
(15��)
��1��ij����Ʒ��װ��ӡ��������ϣ���ѡ���⡢ʳ�Ρ���֬�졢�������ơ��������ڵ�ζ������ ��������ɫ������ �����ڷ��������� ��
��2�����������ж�������ۺ���ζ��ʳ�κ����ƣ����Ҫ��ֹ����ʳ�������ƶ������¹ʡ��������ƺ��Ȼ��ƵIJ����������±���
| ���� | �������� | �Ȼ��� |
| 1�����������µ��ȶ��� | ��ʱ�ֽ�ΪNO��NO2 | ��ʱ���ֽ� |
| 2���۵� | 271�� | 801�� |
| 3������ʱ���ܽ�� | Լ80g | Լ35g |
������ݱ�����Ϣ���һ�ּ���NaNO2��NaCl�ķ�����д���IJ������̡�����ͽ��ۣ�
�ڵ��������в���ȱ�ٵ� ��ѡ���������������Ԫ�ء�
��ʳ���м����Ԫ������Ч��ֹȱ������ļ�������ǰ��ʳ���м���⻯�أ�KI��������һ�����ʧԼ92%������ʳ���м������أ�KIO3��������ͬ�����µ����ʧԼ7%��ʳ���м���⻯�صĵ���ʧ�ʸߵ�ԭ���� ��������ĸ����
a���⻯�ر���������ɵ⻯�صĵ���ʧ�ʸߡ�
b���⻯����������ɵ⻯�صĵ���ʧ�ʸߡ�
c���⻯����ʳ���еijɷַ�����Ӧ����ɵ⻯�صĵ���ʧ�ʸߡ�
����ȡ��ˮ�еĵ�ʱ��һ��ѡ�õ��Լ��ǣ�����ĸ�� ��
A���ƾ� B�����Ȼ�̼ C������
�ݿ��������ữ�ĵ⻯�غ͵�����Һ����ʳ���еĵ���ء���Ӧ��ѧ����ʽΪ��
5KI��KIO3 + 6HCl == 6KCl + 3I2 +3H2O����Ӧ��������
����֪����������ֽ⣬����Ϊ���õ���ؼӵ��ν������ʱӦע��ʲô���⣿ ��
�����ࡢ��֬�������ʶ�����������Ӫ�����ʡ�
����֬�������������ø��������ˮ��Ϊ��֬����� ��д���ƣ����������������ɶ�����̼��ˮ���ṩ����������Ϊ�ϳ����������������ʵ�ԭ�ϡ�
�ڰ���������ɵ����ʵĻ����ṹ��Ԫ���������һ�����еĹ������ǰ�������NH2���� ��д�ṹ��ʽ���������й��ж�ʮ���ְ����ᣬ������������_____����ܡ����ܡ����ϳɵİ������Ϊ������谱���ᡣ
�۵����ڵ���ø������������ˮ��Ϊ�����ǣ�C6H12O6�������������������ڱ��������ɶ�����̼��ˮ��д�������������ڱ������Ļ�ѧ����ʽ�� ��
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(15��)��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݡ�
��.�������A-D��ʾ�����ʣ����¹�����Ҫ������Ũ�������Щ���ʣ��뽫ѡ����ĸ�������и�С��������ڣ�
Aǿ���� B ��ˮ�� C ��ˮ�� D ǿ������
��1��Ũ������Ը��������� ��
��2��Ũ����ʹľ����ڣ� ��
��3���ȵ�Ũ������ͭƬ��Ӧ�� ��
�����ø�Ũ��������100 mL��1 mol/L��ϡ���ᡣ�ɹ�ѡ�õ������У��ٽ�ͷ�ιܣ�����ƿ�����ձ����� ҩ�ף�����Ͳ����������ƽ��
��ش��������⣺
(1)����ϡ����ʱ�����������в���Ҫʹ�õ��� ��ѡ����ţ�����ȱ�ٵ�������
���� ��д�������ƣ���
(2)�����㣬����100mL1mol/L��ϡ������Ҫ����Ͳ��ȡ����Ũ��������Ϊ �� mL������һλС��������ȡŨ����ʱӦѡ�� ��ѡ���10mL����50mL ����100mL��������Ͳ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ�꽭��ʡ����е�һ��ѧ�߶�ѧҵˮƽ���Ի�ѧ�Ծ� ���ͣ������
(15��)
��1��ij����Ʒ��װ��ӡ��������ϣ���ѡ���⡢ʳ�Ρ���֬�졢�������ơ��������ڵ�ζ������ ��������ɫ������ �����ڷ��������� ��
��2�����������ж�������ۺ���ζ��ʳ�κ����ƣ����Ҫ��ֹ����ʳ�������ƶ������¹ʡ��������ƺ��Ȼ��ƵIJ����������±���
| ���� | �������� | �Ȼ��� |
| 1�����������µ��ȶ��� | ��ʱ�ֽ�ΪNO��NO2 | ��ʱ���ֽ� |
| 2���۵� | 271�� | 801�� |
| 3������ʱ���ܽ�� | Լ80g | Լ35g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ����и߶�ѧҵˮƽ���Ի�ѧ�Ծ� ���ͣ������
(15��)
��1��ij����Ʒ��װ��ӡ��������ϣ���ѡ���⡢ʳ�Ρ���֬�졢�������ơ��������ڵ�ζ������ ��������ɫ������ �����ڷ��������� ��
��2�����������ж�������ۺ���ζ��ʳ�κ����ƣ����Ҫ��ֹ����ʳ�������ƶ������¹ʡ��������ƺ��Ȼ��ƵIJ����������±���
|
���� |
�������� |
�Ȼ��� |
|
1�����������µ��ȶ��� |
��ʱ�ֽ�ΪNO��NO2 |
��ʱ���ֽ� |
|
2���۵� |
271�� |
801�� |
|
3������ʱ���ܽ�� |
Լ80g |
Լ35g |
������ݱ�����Ϣ���һ�ּ���NaNO2��NaCl�ķ�����д���IJ������̡�����ͽ��ۣ�
�ڵ��������в���ȱ�ٵ� ��ѡ���������������Ԫ�ء�
��ʳ���м����Ԫ������Ч��ֹȱ������ļ�������ǰ��ʳ���м���⻯�أ�KI��������һ�����ʧԼ92%������ʳ���м������أ�KIO3��������ͬ�����µ����ʧԼ7%��ʳ���м���⻯�صĵ���ʧ�ʸߵ�ԭ���� ��������ĸ����
a���⻯�ر���������ɵ⻯�صĵ���ʧ�ʸߡ�
b���⻯����������ɵ⻯�صĵ���ʧ�ʸߡ�
c���⻯����ʳ���еijɷַ�����Ӧ����ɵ⻯�صĵ���ʧ�ʸߡ�
����ȡ��ˮ�еĵ�ʱ��һ��ѡ�õ��Լ��ǣ�����ĸ�� ��
A���ƾ� B�����Ȼ�̼ C������
�ݿ��������ữ�ĵ⻯�غ͵�����Һ����ʳ���еĵ���ء���Ӧ��ѧ����ʽΪ��
5KI��KIO3 + 6HCl == 6KCl + 3I2 + 3H2O����Ӧ��������
����֪����������ֽ⣬����Ϊ���õ���ؼӵ��ν������ʱӦע��ʲô���⣿ ��
�����ࡢ��֬�������ʶ�����������Ӫ�����ʡ�
����֬�������������ø��������ˮ��Ϊ��֬����� ��д���ƣ����������������ɶ�����̼��ˮ���ṩ����������Ϊ�ϳ����������������ʵ�ԭ�ϡ�
�ڰ���������ɵ����ʵĻ����ṹ��Ԫ���������һ�����еĹ������ǰ�������NH2���� ��д�ṹ��ʽ���������й��ж�ʮ���ְ����ᣬ������������_____����ܡ����ܡ����ϳɵİ������Ϊ������谱���ᡣ
�۵����ڵ���ø������������ˮ��Ϊ�����ǣ�C6H12O6�������������������ڱ��������ɶ�����̼��ˮ��д�������������ڱ������Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���Ĵ�ʡ�ɶ�����УЭ���߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
�v15�֩w��1��������������Ĺ�����������Ӧ�����������������
��������CH2 =CH2��CH3 CH3 ��Ӧѡ�� __ ������ĸ����
a��NaOH��Һ b����ˮ c��������Һ
��������HCHO��HCOOH��Ӧѡ�� _ (����ĸ)��
a��KMnO4��Һ b��������Һ c��Na2CO3��Һ
��������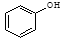 ��
��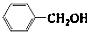 ��Ӧѡ��
___ (����ĸ)��
��Ӧѡ��
___ (����ĸ)��
a��FeCl3��Һ b��NaOH��Һ c��AgNO3��Һ
��2�����л���ѧ�У�ͬ���칹���ձ���ڵ�������ʽΪC4H9OH���л��ﹲ��
�֡����У�һ���л���ͨ����ȥ��Ӧ��ת��Ϊ2-��ϩ����д������ȥ��Ӧ�Ļ�ѧ����ʽ�� ����һ���л��ﲻ�ܷ�������������д�����л���Ľṹ��ʽ�� ��
��3��A��ʯ���ѽ����ijɷ�֮һ��A��ijһͬϵ��E�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�C6H12O2����ϳ�·������ͼ��ʾ��
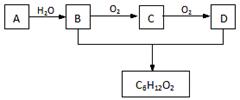
�ش��������⣺
��A�Ľṹ��ʽΪ��______________��
��B��D�����еĹ��������Ʒֱ�Ϊ_______________��_____________��
��д��B��ͬ����ͬ���칹��Ľṹ��ʽ��___________________��
��д��B��C�Ļ�ѧ����ʽ��_________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com