
���� ��1��ԭ�Ӻ����м������ӣ����м����˶�״̬��Na2O2����ˮ��������̼��Ӧ����������
��2��þ�ڶ�����̼�е�ȼ����MgO��C��
��3����ԭ�Ӻ�����3�����Ӳ㣬�������3�����ӣ�
��4����ȥFe�е�����Al������������Һ��
��5��Na��Mg��Al�õ�ⷨұ����Fe��Cu���Ȼ�ԭ��ұ����
��6��ԭ�ӹ���ϵ����Ų�Ϊ��ȫ�ա���������������ȫ������ʱ��һ������ȶ���
��� �⣺��1����ԭ�Ӻ��������11�����ӣ�ԭ�Ӻ����м������ӣ����м����˶�״̬��������ԭ�Ӻ��������11�˶�״̬��Na2O2����ˮ��������̼��Ӧ������������������������
�ʴ�Ϊ��11����������
��2��þ�ڶ�����̼�е�ȼ����MgO��C���䷴Ӧ�Ļ�ѧ����ʽΪ��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
�ʴ�Ϊ��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
��3����ԭ�Ӻ�����3�����Ӳ㣬�������3�����ӣ���AlԪ�������ڱ���λ�ڵ������ڵ�IIIA�壻
�ʴ�Ϊ���������ڵ�IIIA�壻
��4����ȥFe�е�����Al������������Һ��Al��������������Һ��Fe���ܣ��䷴Ӧ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��5��Na��Mg��Al�õ�ⷨұ����Fe��Cu���Ȼ�ԭ��ұ����
�ʴ�Ϊ��Fe��Cu��
��6��ԭ�ӹ���ϵ����Ų�Ϊ��ȫ�ա���������������ȫ������ʱ��һ������ȶ�����̬ͭ��Cu��ԭ�ӵĵ����Ų�ʽΪ[Ar]3d104s1������[Ar]3d94s2������Ϊ[Ar]3d104s1��d�������ȫ����s������ڰ�����
�ʴ�Ϊ����Ϊ[Ar]3d104s1��d�������ȫ����s������ڰ�����
���� ���⿼���˵����Ų�����ѧ����ʽ��Ԫ�����ڱ���������ұ���ȣ���Ŀ�Ѷ��еȣ������ڿ���ѧ���Ի���֪ʶ��Ӧ��������ע����յ����Ų����ɣ�


 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�߶���10���¿���ѧ���������棩 ���ͣ�ѡ����
��ͼ�йص绯ѧ��ʾ��ͼ��ȷ����
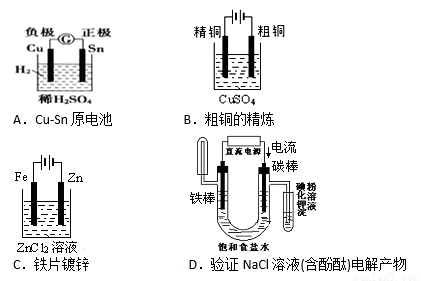
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ��һ��10���¿���ѧ���������棩 ���ͣ�ѡ����
��������ƿ��������������������һ������������Һ���������ڲ���������Һ���۲����������ȣ���ʹ��֮ǰҪ����Ƿ�©ˮ����Щ��������ȷ����
A. �٢ڢۢ� B. �ڢ� C. �٢ڢ� D. �ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ��һ��10���¿���ѧ���������棩 ���ͣ�ѡ����
������ȼ���ױ����ж��Ļ�ѧ���ʣ������������װ����Σ�վ����ǩ���������������У������˱�ǩ����
| A | B | C | D |
���ʵĻ�ѧʽ | H2SO4(Ũ) | C2H5OH(�ƾ�) | Hg(��) | NaCl |
Σ�վ����־ |
|
|
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
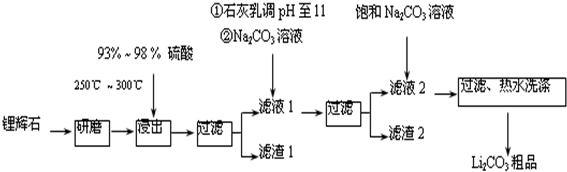
| T/�� | 20 | 40 | 60 | 80 |
| S��Li2CO3��/g | 1.33 | 1.17 | 1.01 | 0.85 |
| S��Li2SO4��/g | 34.2 | 32.8 | 31.9 | 30.7 |
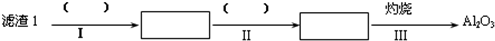
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
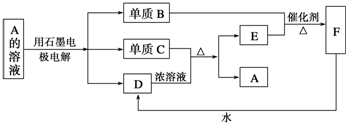
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
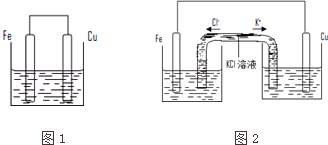
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CHOHCH3 | B�� | CH2OHCH2CH3 | C�� | ��CH3��2COHCH3 | D�� | ��CH3��3COH |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com