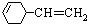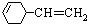��ѧ��ѧ����������A��B��C��D֮���������ת����ϵ��A+B��C+D+H
2O��û����ƽ�����밴Ҫ����գ�
��1����AΪ������Ԫ����ɵĺ�ɫ���嵥�ʣ���B��Ũ��Һ����ʱ������C��D�������壮C��D�����������ʹ����ʯ��ˮ����ǣ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�
C+2H
2SO
4��Ũ��
CO
2��+2SO
2��+2H
2O
C+2H
2SO
4��Ũ��
CO
2��+2SO
2��+2H
2O
���������������岻��ѡ�õ��Լ���
a
a
a��BaCl
2��Һ b��KMnO
4��Һ c��Ʒ����Һ d���ữ��Ba��NO
3��
2��Һ
��500mL2mol/L��NaOH��Һ��ͨ��0.8mol��ɫ��ζ��C���壬ǡ����ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��
5NaOH+4CO2�TNa2CO3+3NaHCO3+H2O
5NaOH+4CO2�TNa2CO3+3NaHCO3+H2O
����ʱ��Һ�е����Ӱ���Ũ���ɴ�С���е�˳����
c��Na+����c��HCO3-����c��CO3 2- ����c��OH-����c��H+��
c��Na+����c��HCO3-����c��CO3 2- ����c��OH-����c��H+��
��
��2����AΪ��ɫ�������ʣ�������B����Һ�ڳ�����ǡ����ȫ��Ӧ�����ɵ���ɫ����C������Ѹ�ٱ�ɾ���ɫ��������ԭ��B���ʵ���Ϊ2molʱ������C����������
44.8
44.8
L��������������ɵĺ���ɫ����ͨ��һ����ƿ�����ƿ������ƿ�����ˮ�У���ƿ���������ɫ��dz�����û�ѧ����ʽ�ͱ�Ҫ�����ֽ�����ɫ�仯��ԭ��
2NO2������ɫ��?N2O4����ɫ������H��0�����£�ƽ��������Ӧ�����ƶ�������ɫNO2Ũ�ȼ�С������������ɫ��dz
2NO2������ɫ��?N2O4����ɫ������H��0�����£�ƽ��������Ӧ�����ƶ�������ɫNO2Ũ�ȼ�С������������ɫ��dz
��
��3����A��ˮ�е��ܽ�����¶ȵ����߶����ͣ�BΪ�����ڷǽ������ʣ�D��Ư�۵ijɷ�֮һ��C����ˮ�ⷴӦ�����ӷ���ʽ��
ClO-+H2O?HClO+OH-
ClO-+H2O?HClO+OH-
��4����AΪ���10���ӵ��������뵥��18���ӵ������ӹ��ɵ���ɫ���壬�����ֽ⣬�ֽ���������ּ�������ˮ�����壮����A�������ӵķ�����
ȡ����A��һ�ྻ�Թ��У�������ˮ�ܽ�μ���������Һ��������ɫû�����ٵμ�ϡ���ᣬ���������ܽ⣬��A��������ΪCl-
ȡ����A��һ�ྻ�Թ��У�������ˮ�ܽ�μ���������Һ��������ɫû�����ٵμ�ϡ���ᣬ���������ܽ⣬��A��������ΪCl-
�������������̼����ۣ���



 ������O��O�������еĦм���ĿΪ2��
������O��O�������еĦм���ĿΪ2�� ��2��
��2��

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�


 +2Ag��NH3��2OH
+2Ag��NH3��2OH +2Ag+3NH3+H2O
+2Ag+3NH3+H2O +2Ag��NH3��2OH
+2Ag��NH3��2OH +2Ag+3NH3+H2O
+2Ag+3NH3+H2O

 ��
��
 ��
��