
ijͬѧ��̽�����̼ԭ�ӵĵõ���������ǿ����ͨ���Ƚ���������������Ӧˮ���������ǿ������֤���������ͼʵ�飬��ش�
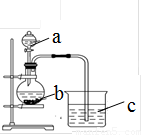
��1������a�������� ��Ӧʢ������ҩƷ�е� ������ţ���
A��ϡ���� B. ������ C. ������ D. ����
��2������bӦʢ������ҩƷ�е� ������ţ���
A��̼��� B. ������ C. �Ȼ��� D. ̼����
��3��b�з�Ӧ�����ӷ���ʽΪ ��
����������C�е������� ������֤�� �� ����ǿ���ѧʽ�����õ��ӵ����� �� ǿ��
��1����Һ©����A��2��D��3��CO32����2H����H2O��CO2����2�֣�
����ʯ��ˮ�����
�� ��
��  �� �� �� ̼ ����1�֣�
�� �� �� ̼ ����1�֣�
��������
�����������1�����������Ľṹ�ص��֪��a�Ƿ�Һ©����Ҫ�Ƚ���������������Ӧˮ���������ǿ��������ݽ�ǿ�����Ʊ�������ԭ����֪��a��ʢ�ŵ�ҩƷ��ϡ���ᣬ��ѡA����2�����ݣ�1���еķ�����֪��b��ʢ�ŵ���̼���Ρ�������������������ʣ�����ѡ�����̼���ƣ���ѡD����3��b���Ʊ�CO2�ģ���Ӧ�����ӷ���ʽ��CO32����2H����H2O��CO2����CO2��ʹ����ʯ��ˮ����ǣ���C�е�ʵ�������dz���ʯ��ˮ����ǣ��ݴ˿�֪�����̼��ǿ��S�ĵõ����ӵ�����ǿ��̼ԭ�ӵġ�
���㣺�������е��Ѷȵ����⣬���������ǿ���������У�����������ѧ���������������淶�Ͻ���ʵ���������������������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ���������֪ʶ���ʵ�������������


 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����һ��ѧ����1�¡�ԭ�ӽṹ��Ԫ�������ɡ�1.3.2Ԥ��ͬ����Ԫ�ص�����(³�ư����2) ���ͣ�058
ijͬѧ��ͨ��ʵ��̽�����̼�õ���������ǿ����ͨ���Ƚ���������������Ӧˮ���������ǿ������֤���������ͼʵ�飮
����������⣺
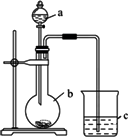
(1)����a��������________��Ӧʢ������ҩƷ�е�________��
A��ϡ����
B��������
C��������
D������
(2)����b��������________��Ӧʢ������ҩƷ�е�________��
A��̼���
B��������
C���Ȼ���
D��̼����
(3)����c��Ӧʢ�ŵ���Ʒ��________�����������������________________��֤��b�з�Ӧ������________������֤��________��________����ǿ���õ���������ǿ��b�з�����Ӧ�����ӷ���ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ�����п���ѧУ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
ijͬѧ��̽�����̼ԭ�ӵĵõ���������ǿ����ͨ���Ƚ���������������Ӧˮ���������ǿ������֤���������ͼʵ�飬��ش�
��1������a�������� ��
Ӧʢ������ҩƷ�е� ������ţ���
A��ϡ���� B. ������ C. ������ D. ����
��2������bӦʢ������ҩƷ�е� ������ţ���
A��̼��� B. ������ C. �Ȼ��� D. ̼����
��3��b�з�Ӧ�����ӷ���ʽΪ ��
����������C�е������� ������֤�� �� ����ǿ���ѧʽ�����õ��ӵ����� �� ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ�����п���ѧУ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
ijͬѧ��̽�����̼ԭ�ӵĵõ���������ǿ����ͨ���Ƚ���������������Ӧˮ���������ǿ������֤���������ͼʵ�飬��ش�

��1������a�������� ��
Ӧʢ������ҩƷ�е� ������ţ���
A��ϡ���� B. ������ C. ������ D. ����
��2������bӦʢ������ҩƷ�е� ������ţ���
A��̼��� B. ������ C. �Ȼ��� D. ̼����
��3��b�з�Ӧ�����ӷ���ʽΪ ��
����������C�е������� ������֤�� �� ����ǿ���ѧʽ�����õ��ӵ����� �� ǿ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com