
���������ھ�������������ˮ��������������ҵ�������Ư����
��1���������������������ҿ������������е��ʷ�Ӧ���磺6Ag��s����O3��g��=3Ag2O��s������H����235.8 kJ��mol��1����֪2Ag2O��s��=4Ag��s����O2��g�� ��H����62.2 kJ��mol��1�������·�Ӧ2O3��g��=3O2��g���Ħ�H��________��
��2����ƽ���淴Ӧ�Ļ�ѧ����ʽ���������ʵĻ�ѧ������������Ӧ�ķ����ڣ���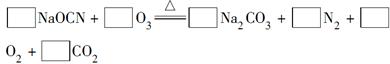
��3����ѧ��P.Tatapudi��������ʹ�������������µ��ˮ�ķ����Ƶó�����������������Χ��ˮ�в������缫��ӦʽΪ3H2O��6e��=O3����6H�������������ܽ���ˮ�е��������ɹ������⣬��缫��ӦʽΪ_______________________��
��4�������г����ļ�ⷽ���ǽ���������ͨ������KI������Һ������Һ����ɫ����˵�������к���O3����֪O3��KI��Һ��Ӧ�������ֵ��ʣ���÷�Ӧ�����ӷ���ʽΪ_____________________________________________________��
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ԭ��Ӧ�������������о��й㷺����;���ᴩ�Ž�
(1)ˮ���������Ҫ��ɳɷ֣��������к�������һ�����ʡ��������ֻ�����Ӧ����
��������ԭ��Ӧ�Ĺ�ϵ��Ҳ������ͼ���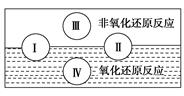
��д����ˮ�μӵķ��Ϸ�Ӧ���͢���һ����ѧ����ʽ��________________________������ˮΪ________����
(2)�Ȼ�麟��������ӡ��磺�ں���ͭ��ʱ���Ȼ�麟�ȥͭ�����������ͭ�Ա㺸�ӣ��䷴ӦΪ��
CuO��____NH4Cl Cu��____CuCl2��N2����____H2O��
Cu��____CuCl2��N2����____H2O��
����ƽ��������ԭ��Ӧ����ʽ��
�ڸ÷�Ӧ�У���������Ԫ����________(��Ԫ������)����������________(�ѧʽ)��
(3)(2011�������߿�����ѡ)������뽹̿��ʯӢɰ��ϣ��ڵ�¯�м��ȵ�1 500 �����ɰ��ף���ӦΪ��
2Ca3(PO4)2��6SiO2=6CaSiO3��P4O10��10C��P4O10=P4��10CO��
ÿ����1 mol P4ʱ������________mol���ӷ���ת�ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ԭ��Ӧ��ʵ���ϰ��������ͻ�ԭ�������̡�������һ����ԭ���̵ķ�Ӧʽ��NO��4H����3e��=NO����2H2O��KMnO4��Na2CO3��Cu2O��Fe2(SO4)3���������е�һ������(��)��ʹ������ԭ���̷�����
��1��д������ƽ��������ԭ��Ӧ�ķ���ʽ��__________________________________��
��2����Ӧ������������______________��______________�����ʡ�
��3����Ӧ��������0.2 mol���壬��ת�Ƶĵ��ӵ����ʵ�����________ mol��
��4����1 mol����ijŨ�����ᷴӦʱ������ԭ��������ʵ������ӣ�ԭ����________________________________________________________________________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����г������ؽ�����Ⱦ���У�����Ǧ���̡������ӡ�������ҵ��ˮ�к��е�Cr2O72-��CrO42-�����õķ��������֡�
����1����ԭ������
�÷��Ĺ�������Ϊ ��
��
���еڢٲ�����ƽ��2CrO42-����ɫ����2H�� Cr2O72-����ɫ����H2O��
Cr2O72-����ɫ����H2O��
��1��д���ڢٲ���Ӧ��ƽ�ⳣ������ʽ_________________________________��
��2�����ڵڢٲ���Ӧ������˵����ȷ����________��
A��ͨ���ⶨ��Һ��pH�����жϷ�Ӧ�Ƿ��Ѵ�ƽ��״̬
B���÷�ӦΪ������ԭ��Ӧ
C��ǿ���Ի�������Һ����ɫΪ��ɫ
��3���ڢڲ��У���ԭ0.1 mol Cr2O72-����Ҫ________mol��FeSO4��7H2O��
��4���ڢ۲�������Cr��OH��3�⣬���������ɵij���Ϊ________������Һ�д������³����ܽ�ƽ�⣺Cr��OH��3��s�� Cr3����aq����3OH����aq���������£�Cr��OH��3���ܶȻ�Ksp��10��32����c��Cr3��������10��5 mol��L��1ʱ����Ϊc��Cr3�����Ѿ���ȫ�������ֽ��ڢ۲���Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ����д��������̣���____________________________________________________________________________��
Cr3����aq����3OH����aq���������£�Cr��OH��3���ܶȻ�Ksp��10��32����c��Cr3��������10��5 mol��L��1ʱ����Ϊc��Cr3�����Ѿ���ȫ�������ֽ��ڢ۲���Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ����д��������̣���____________________________________________________________________________��
����2����ⷨ
��5��ʵ����������ͼװ��ģ���ⷨ������Cr2O72-�ķ�ˮ�����ʱ������ӦʽΪ________��������ӦʽΪ________���õ��Ľ������������������ɳ�����ȫ����ˮ�ĵ���ƽ��ǶȽ�����ԭ����___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������(H2O2)��һ����ɫ��Һ��,����ˮ��Һ�׳�˫��ˮ,��������,����������������ɱ������Ư���ȡ�
(1)����˵����ȷ���� ��
| A��������������м��м��Լ����зǼ��Լ� |
| B��H2O2��H2O��Ϊͬ�������� |
| C��34 g H2O2�к��е���������ΪNA |
| D��ʵ���ҿ������ù���������ȡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������[��NH4��2Ce��NO3��6]�㷺Ӧ���ڵ��ӡ�����ҵ����ϳ�·�����£�
��1����֪��NH4��2Ce��NO3��6�����ֽ⣬ij����С����Ϊ��Ӧԭ�����£��벹����������NH4��2Ce��NO3��6 CeO2��8OH + 8_____����CeO2��8OH
CeO2��8OH + 8_____����CeO2��8OH CeO2+ 4H2O��+2O2����
CeO2+ 4H2O��+2O2����
�ڿ����м��ȣ�NH4��2Ce��NO3��6����������ɫ�б仯�⣬���ɹ۲쵽��������_________��
��2��������У���Ce��NO3��3��6H2O�����Һ������pH��4~5����������H2O2��Һ�����Ͼ��ȣ��ټ��백ˮ������ҺpH���õ�Ce��OH��4�������ù����вμӷ�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ___________��
��3��298Kʱ��Ksp[Ce��OH��4]��1��10��29��Ce��OH��4���ܶȻ�����ʽΪKsp��___________��
Ϊ��ʹ��Һ��Ce4��������ȫ������������Һ�е�c��Ce4+��С��1��10��5mol��L��1�������pHΪ______���ϡ�
��4��Ϊ���о������Ĺ�������������С��ⶨ�ˣ�NH4��2Ce��NO3��6�ڲ�ͬ�¶ȡ���ͬŨ�������е��ܽ�ȣ������ͼ����ͼ�пɵó�������Ҫ���ɣ�
�� ��NH4��2Ce��NO3��6�������е��ܽ�����¶����߶�����
�� _____________________________________________��
�� _____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����������Ҫ�Ĺ�ҵԭ�ϡ�
�ٿ��������������Ũ����������� ��
��ij���᳧����β��NO2�ķ����ǣ���������ʱ��H2��NO2��ԭΪN2��
��֪��2H2(g) + O2(g) �� 2H2O(g) ��H����483 kJ��moL��1
N2(g) + 2O2(g) �� 2NO2(g) ��H����68 kJ��moL��1
��H2��ԭNO2����ˮ�������Ȼ�ѧ����ʽ�ǣ�
��
��2��ij�о�С����CaCl2��H2Ϊԭ���Ʊ�+1��Ca�Ļ����������ֻ�м������ֻ�����о����֣������������иơ���Ԫ�ص����������ֱ�Ϊ52.29%��46.41%���������ҵ�ˮ��Һ�����ԡ���ش��������⣺
���ҵĻ�ѧʽΪ ������ˮ��Ӧ�ɵ�H2���仯ѧ����ʽ�ǣ� ��
��д����CaCl2ͨ�����Ϸ�Ӧ�Ʊ�CaCl�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ƫ��������N2O4�dz��õĻ���ƽ��������߷������»�ѧ��Ӧ��
(CH3)2NNH2 (l)��2N2O4 (l)��2CO2 (g)��3N2(g)��4H2O(g) ��I��
(1)��Ӧ��I������������_______��
(2)����к��г��ֺ���ɫ���壬ԭ��Ϊ��N2O4 (g)  2NO2 (g) ���� һ���¶��£���Ӧ�����ʱ�Ϊ��H���ֽ�1 mol N2O4 ����һ��ѹ�ܱ������У�����ʾ��ͼ��ȷ����˵����Ӧ�ﵽƽ��״̬����________��
2NO2 (g) ���� һ���¶��£���Ӧ�����ʱ�Ϊ��H���ֽ�1 mol N2O4 ����һ��ѹ�ܱ������У�����ʾ��ͼ��ȷ����˵����Ӧ�ﵽƽ��״̬����________��
������ͬ�¶��£�������Ӧ�������Ϊ1L�ĺ����ܱ������н��У�ƽ�ⳣ��________����������䡱��С��������Ӧ3s��NO2�����ʵ���Ϊ0.6mol����0��3s�ڵ�ƽ����Ӧ����v��N2O4����________mol��L-1��s-1��
(3)NO2���ð�ˮ��������NH4NO3��25��ʱ����amol NH4NO3����ˮ����Һ�����ԣ�ԭ����_____
_______________________________________________�������ӷ���ʽ��ʾ���������Һ�μ�bL��ˮ����Һ�����ԣ���μӰ�ˮ�Ĺ����е�ˮ�ĵ���ƽ�⽫______������������������ƶ������μӰ�ˮ��Ũ��Ϊ_______mol��L-1����NH3��H2O�ĵ���ƽ�ⳣ��ȡKb��2��10-5 mol��L-1��������Һ�������bL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
I��1���ڵ��۵⻯����Һ��ͨ�����������������ῴ����Һ����ɫ����Ӧ�����ӷ���ʽ�� ��
��2���ڵ�͵����γɵ���ɫ��Һ��ͨ��SO2���壬������ɫ����ʧ����Ӧ�����ӷ����� ��
��3���Աȣ�1���ͣ�2��ʵ�����õĽ������Cl ��I
��I SO2����ԭ����ǿ����˳������Ϊ ��
SO2����ԭ����ǿ����˳������Ϊ ��
II ��4�� ��ȥ�����л������۵��Լ��� �����ӷ���ʽΪ
��5�� 1mol����������2mol̼�����ƹ����Ϻ����ܱ������м��ȳ�ַ�Ӧ���ų��������ʺ���ȴ�������Ĺ���������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com