
 ��
�� ��Fe2+�ͽᾧˮ����������в������裺��ȡ�����û�������ȣ������Թܿڴ���Һ�壬��Һ����ʹ��ˮ����ͭ����������ȡ�����û���������ˮ�У��μ���������ˮ���ټ���KSCN��Һ����Ѫ��ɫ���֣���ȡ������Һ���Թ��У��������ᣬû�������ټ���BaCl2��Һ���а�ɫ�������ɣ���ȡ������Һ���Թ��У�����Ũ��NaOH��Һ������ʹʪ��ĺ�ɫʯ����ֽ���������������ͬʱҲ�а�ɫ�����������ó����ܿ��ɻ���ɫ������
��Fe2+�ͽᾧˮ����������в������裺��ȡ�����û�������ȣ������Թܿڴ���Һ�壬��Һ����ʹ��ˮ����ͭ����������ȡ�����û���������ˮ�У��μ���������ˮ���ټ���KSCN��Һ����Ѫ��ɫ���֣���ȡ������Һ���Թ��У��������ᣬû�������ټ���BaCl2��Һ���а�ɫ�������ɣ���ȡ������Һ���Թ��У�����Ũ��NaOH��Һ������ʹʪ��ĺ�ɫʯ����ֽ���������������ͬʱҲ�а�ɫ�����������ó����ܿ��ɻ���ɫ������| A�����е�����˵��һ����Fe2+ |
B��Ϊ�˼��� �����Խ����е��Լ���ΪHNO3�ữ��Ba(NO3)2 �����Խ����е��Լ���ΪHNO3�ữ��Ba(NO3)2 |
| C��ͨ������ʵ���ȷ���û�����Ļ�ѧʽΪ��NH4��2Fe(SO4)2��6H2O |
| D�����еij�����ʱ����û��ɺ��ɫ���� |
 �������HNO3�ữ��Ba(NO3)2,��
�������HNO3�ữ��Ba(NO3)2,�� Ҳ�������������˵���û������к�
Ҳ�������������˵���û������к� �����е�����˵���û������к�Fe2+��
�����е�����˵���û������к�Fe2+�� ������ӦΪFe(OH)2,Fe(OH)2���ױ������е�O2�����ɺ��ɫ����Fe(OH)3��ͨ����������ֻ��ȷ���û������к�Fe2+��
������ӦΪFe(OH)2,Fe(OH)2���ױ������е�O2�����ɺ��ɫ����Fe(OH)3��ͨ����������ֻ��ȷ���û������к�Fe2+�� ��
�� �ͽᾧˮ������ȷ���仯ѧʽ����ֻ��D��ȷ��
�ͽᾧˮ������ȷ���仯ѧʽ����ֻ��D��ȷ��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


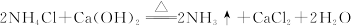
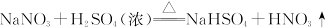
| A���٢ڢۢ� |
| B���� |
| C���ڢ� |
| D��ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| a/g | ��ʼ����/mL | b/mL | c/mL |
| 0.1970 | 0.00 | 31.30 | 42.40 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| Na2CO3 | NaHCO3 | NaCl | Na2SO4 | NaHSO4 | NaOH |
| 15.9 | 8.4 | 35 | 35.5 | 20 | 40 |
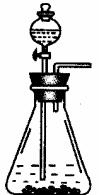
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
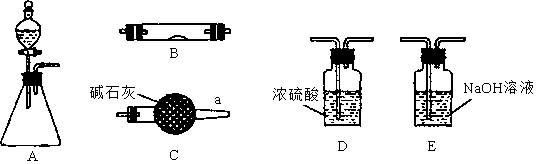
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 K2C2O4
K2C2O4�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CO2 | B��H2 | C��NO2 | D��NO |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com