
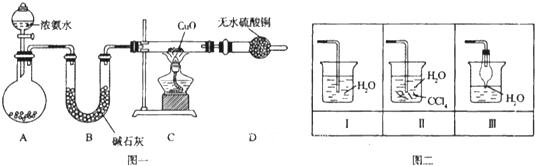
£Ø12·Ö£©Ä³»ÆѧŠĖȤŠ”×éĄūÓĆĻĀĶ¼ĖłŹ¾Ōµē³Ų×°ÖĆ½ųŠŠŹµŃ飬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŹµŃéÖŠ£¬Ķ¬Ń§ĆĒ·¢ĻÖĮ½×°ÖƵēĮ÷¼ĘµÄÖøÕėĘ«×Ŗ·½Ļņ²»Ķ¬£¬Ņņ“ĖÓŠČĖĢį³öŅŌĻĀ¹Ūµć£¬ĘäÖŠÕżČ·µÄŹĒ____________”££ØĖ«Ń”Ģā£¬Ā©Ń”µĆ1·Ö£¬¶ąŃ”“ķŃ”µĆ0·Ö£©
| A£®½šŹō»ī¶ÆŠŌĀĮ±ČĆ¾Ēæ |
| B£®½šŹō»ī¶ÆŠŌĆ¾±ČĀĮĒ棬Į½×°ÖĆÖŠĆ¾¾łĪŖøŗ¼« |
| C£®½öøł¾Ż½šŹō»ī¶ÆŠŌĖ³Šņ²»ÄÜ×¼Č·ÅŠ¶ĻŌµē³ŲµÄÕżøŗ¼« |
| D£®Ōµē³ŲÖŠµÄÕżøŗ¼«ŹÜµē½āÖŹČÜŅŗµÄĖį¼īŠŌ”¢ĒæŃõ»ÆŠŌµČŅņĖŲµÄÓ°Ļģ |
 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģɽ¶«Ź”ČÕÕÕŹŠøßČż12ŌĀŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø11·Ö£©Ä³»ÆѧŠĖȤŠ”×éĄūÓĆŅŌĻĀ×°ÖĆĢ½¾æĀČĘųÓė°±ĘųÖ®¼äµÄ·“Ó¦”£ĘäÖŠA”¢F·Ö±šĪŖ°±ĘųŗĶĀČĘųµÄ·¢Éś×°ÖĆ£¬CĪŖ“æ¾»øÉŌļµÄĀČĘųÓė°±Ęų·¢Éś·“Ó¦µÄ×°ÖĆ”£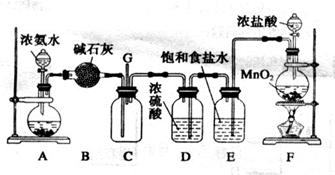
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©×°ÖĆAÖŠµÄ¹ĢĢåĪļÖŹ²»ŅĖŃ”ÓĆ_______________£ØĢī×ÖÄø±ąŗÅ£©”£
a.ĪŽĖ®ĀČ»ÆøĘ b.ĒāŃõ»ÆÄĘ c.Ńõ»ÆøĘ d.¼īŹÆ»Ņ e.ĪŽĖ®ĮņĖįĶ
£Ø2£©Š“³ö×°ÖĆFÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
_______________________________________________________________________£»
E×°ÖƵÄ ×÷ÓĆĪŖ________________________________________________________”£
×÷ÓĆĪŖ________________________________________________________”£
£Ø3£©ĶØČėC×°ÖƵÄĮ½øłµ¼ ¹Ü×ó±ß½Ļ³¤”¢ÓŅ±ß½Ļ¶Ģ£¬ŌŅņŹĒ________________________
¹Ü×ó±ß½Ļ³¤”¢ÓŅ±ß½Ļ¶Ģ£¬ŌŅņŹĒ________________________
___________________________________________________________________________ӣ
£Ø4£©×°ÖĆCÄŚ³öĻÖÅØŗńµÄ°×ŃĢ²¢ŌŚČŻĘ÷ÄŚ±ŚÄż½į£¬ĮķŅ»Éś³ÉĪļŹĒæÕĘųµÄÖ÷ŅŖ³É·ÖÖ®Ņ»”£µ±ÓŠ0.15molCl2²ĪÓė·“Ó¦Ź±£¬ŌņÉś³ÉµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ____________”£
£Ø5£©ÉĻŹö×°ÖĆ»¹“ęŌŚŅ»“¦Ć÷ĻŌµÄȱĻŻ£¬ÄćČĻĪŖøĽųµÄ“ėŹ©ŹĒ____________________
___________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013Ń§ÄźÉ½¶«Ź”µĀÖŻŹŠŌ¾»ŖѧøßČż12ŌĀŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
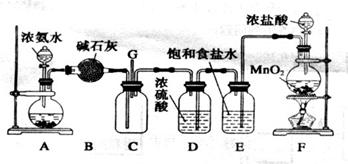
£Ø11·Ö£©Ä³»ÆѧŠĖȤŠ”×éĄūÓĆŅŌĻĀ×°ÖĆĢ½¾æĀČĘųÓė°±ĘųÖ®¼äµÄ·“Ó¦”£ĘäÖŠA”¢F·Ö±šĪŖ°±ĘųŗĶĀČĘųµÄ·¢Éś×°ÖĆ£¬CĪŖ“æ¾»øÉŌļµÄĀČĘųÓė°±Ęų·¢Éś·“Ó¦µÄ×°ÖĆ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©×°ÖĆAÖŠµÄ¹ĢĢåĪļÖŹ²»ŅĖŃ”ÓĆ_______________£ØĢī×ÖÄø±ąŗÅ£©”£
a.ĪŽĖ®ĀČ»ÆøĘ b.ĒāŃõ»ÆÄĘ c.Ńõ»ÆøĘ d.¼īŹÆ»Ņ e.ĪŽĖ®ĮņĖįĶ
£Ø2£©Š“³ö×°ÖĆFÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
_______________________________________________________________________£»
E×°ÖƵÄ×÷ÓĆĪŖ________________________________________________________”£
£Ø3£©ĶØČėC×°ÖƵÄĮ½øłµ¼¹Ü×ó±ß½Ļ³¤”¢ÓŅ±ß½Ļ¶Ģ£¬ŌŅņŹĒ________________________
___________________________________________________________________________ӣ
£Ø4£©×°ÖĆCÄŚ³öĻÖÅØŗńµÄ°×ŃĢ²¢ŌŚČŻĘ÷ÄŚ±ŚÄż½į£¬ĮķŅ»Éś³ÉĪļŹĒæÕĘųµÄÖ÷ŅŖ³É·ÖÖ®Ņ»”£µ±ÓŠ0.15molCl2²ĪÓė·“Ó¦Ź±£¬ŌņÉś³ÉµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ____________”£
£Ø5£©ÉĻŹö×°ÖĆ»¹“ęŌŚŅ»“¦Ć÷ĻŌµÄȱĻŻ£¬ÄćČĻĪŖøĽųµÄ“ėŹ©ŹĒ____________________
___________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com