
(7·Ö)ĻĀĶ¼±ķŹ¾µÄ·“Ó¦¹ŲĻµÖŠ£¬²æ·Ö²śĪļ±»ĀŌČ„”£ŅŃÖŖ2mol°×É«¹ĢĢå·ŪÄ©XŹÜČČ·Ö½ā£¬»Öø“µ½ŹŅĪĀÉś³É°×É«¹ĢĢåA.ĪŽÉ«ŅŗĢåB.ĪŽÉ«ĘųĢåCø÷1mol”£X.E.GµÄŃęÉ«·“Ó¦¾łĪŖ»ĘÉ«”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Š“³öĻĀĮŠĪļÖŹµÄ»ÆѧŹ½£ŗG D
(2)Š“³öGÓėC·“Ӧɜ³ÉDµÄ»Æѧ·“Ó¦·½³ĢŹ½£ŗ
(3)Š“³öX£«E![]() AµÄĄė×Ó·½³ĢŹ½£ŗ
AµÄĄė×Ó·½³ĢŹ½£ŗ
(4)Š“³öCÓė![]() ²Ī¼Ó·“Ó¦µÄ»Æѧ·½³ĢŹ½______________________________________£¬
²Ī¼Ó·“Ó¦µÄ»Æѧ·½³ĢŹ½______________________________________£¬
Čō0.2mol![]() ²Ī¼Ó·“Ó¦,Ōņ×ŖŅʵĵē×ÓŹżĪŖ_____________øö”£
²Ī¼Ó·“Ó¦,Ōņ×ŖŅʵĵē×ÓŹżĪŖ_____________øö”£
(5)Š“³öĪļÖŹXµÄÓĆĶ¾£ØÖĮÉŁŠ“³öŅ»ÖÖ£©______________________________________
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģĢģ½ņŹŠ“óøŪĒųµŚŅ»ÖŠŃ§øßČżµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
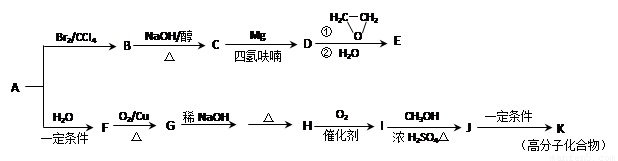
£Ø15·Ö£©ĢžAŹĒŅ»ÖÖÖŲŅŖµÄ»ł±¾»Æ¹¤ŌĮĻ£¬ÓĆÖŹĘ׷زāµĆĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ28”£ĻĀĶ¼ŹĒŅŌAĪŖŌĮĻŗĻ³ÉŅ©ĪļÖŠ¼äĢåEŗĶŹ÷Ö¬KµÄĀ·Ļß”£
ŅŃÖŖ£ŗI. 
II. 
£ØR”¢R”ƱķŹ¾Ģž»ł»ņĒāŌ×Ó£©
£Ø1£©AÖŠ¹ŁÄÜĶŵĽį¹¹¼ņŹ½ŹĒ ”£ÓŠ»śĪļBµÄĆū³Ę
£Ø2£©B”śCµÄ»Æѧ·½³ĢŹ½ĪŖ ”£BŗĶĒāŃõ»ÆÄʵÄĖ®ČÜŅŗ¼ÓČČ·“Ó¦ĖłµĆµ½µÄÓŠ»ś²śĪļŗĶŅŅ¶žĖį·“Ӧɜ³Éøß·Ö×Ó»ÆŗĻĪļ£¬Š“³öÉś³Éøß·Ö×Ó»ÆŗĻĪļ·“Ó¦µÄ»Æѧ·½³ĢŹ½
£Ø3£©EµÄ·Ö×ÓŹ½ĪŖC4H8O”£ĻĀĮŠ¹ŲÓŚEµÄĖµ·ØÕżČ·µÄŹĒ £ØĢī×ÖÄøŠņŗÅ£©”£
a. ÄÜÓė½šŹōÄĘ·“Ó¦ b. ·Ö×ÓÖŠ4øöĢ¼Ō×ÓŅ»¶Ø¹²Ę½Ćę
c. Ņ»¶ØĢõ¼žĻĀ£¬ÄÜÓėÅØĒāäåĖį·“Ó¦ d. ÓėCH2=CHCH2OCH2CH3»„ĪŖĶ¬ĻµĪļ
£Ø4£©G”śHÉę¼°µ½µÄ·“Ó¦ĄąŠĶÓŠ ”£
£Ø5£©IµÄ·Ö×ÓŹ½ĪŖC4H6O2£¬Ęä½į¹¹¼ņŹ½ĪŖ ”£
£Ø6£©J”śKµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø7£©Š“³öÓėE¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄĖłÓŠĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ
£Ø²»æ¼ĀĒĖ³·“Ņģ¹¹£¬²»æ¼ĀĒ”ŖOHĮ¬ŌŚĖ«¼üĢ¼ÉĻµÄ½į¹¹£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğÕć½Ź”Äž²ØŹŠŹ®Š£ĮŖæ¼Ąķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
Įņ“śĮņĖįÄĘ£ØNa2S2O3£©æÉÓÉŃĒĮņĖįÄĘŗĶĮņ·ŪĶعż»ÆŗĻ·“Ó¦ÖĘµĆ£ŗNa2SO3 + S  Na2S2O3£¬³£ĪĀĻĀČÜŅŗÖŠĪö³ö¾§ĢåĪŖNa2S2O3·5H2O”£Na2S2O3”¤5H2OÓŚ40”«45”ęČŪ»Æ£¬48”ę·Ö½ā£»Na2S2O3 Ņ×ČÜÓŚĖ®£¬²»ČÜÓŚŅŅ“¼”£ŌŚĖ®ÖŠÓŠ¹ŲĪļÖŹµÄČܽā¶ČĒśĻßČēÓŅĶ¼ĖłŹ¾”£
Na2S2O3£¬³£ĪĀĻĀČÜŅŗÖŠĪö³ö¾§ĢåĪŖNa2S2O3·5H2O”£Na2S2O3”¤5H2OÓŚ40”«45”ęČŪ»Æ£¬48”ę·Ö½ā£»Na2S2O3 Ņ×ČÜÓŚĖ®£¬²»ČÜÓŚŅŅ“¼”£ŌŚĖ®ÖŠÓŠ¹ŲĪļÖŹµÄČܽā¶ČĒśĻßČēÓŅĶ¼ĖłŹ¾”£

¢ń£®ĻÖ°“ČēĻĀ·½·ØÖʱøNa2S2O3”¤5H2O£ŗ
½«Įņ»ÆÄĘŗĶĢ¼ĖįÄĘ°“·“Ó¦ŅŖĒó±ČĄżŅ»²¢·ÅČėČż¾±ÉÕĘæÖŠ£¬×¢Čė150mLÕōĮóĖ®Ź¹ĘäČܽā£¬ŌŚ·ÖŅŗĀ©¶·ÖŠ£¬×¢ČėÅØŃĪĖį£¬ŌŚ×°ÖĆ2ÖŠ¼ÓČėŃĒĮņĖįÄĘ¹ĢĢ壬²¢°“ĻĀĶ¼°²×°ŗĆ×°ÖĆ”£
£Ø1£©ŅĒĘ÷2µÄĆū³ĘĪŖ £¬
×°ÖĆ6ÖŠæÉ·ÅČė ”£
A£®BaCl2ČÜŅŗ B£®ÅØH2SO4 C£®ĖįŠŌKMnO4ČÜŅŗ D£®NaOHČÜŅŗ

£Ø2£©“ņæŖ·ÖŅŗĀ©¶·»īČū£¬×¢ČėÅØŃĪĖįŹ¹·“Ó¦²śÉśµÄ¶žŃõ»ÆĮņĘųĢå½Ļ¾łŌȵÄĶØČėNa2SŗĶNa2CO3µÄ»ģŗĻČÜŅŗÖŠ£¬²¢ÓĆ“ÅĮ¦½Į°čĘ÷½Į¶Æ²¢¼ÓČČ£¬·“Ó¦ŌĄķĪŖ£ŗ
¢ŁNa2CO3+SO2 =Na2SO3+CO2
¢ŚNa2S+SO2+H2O=Na2SO3+H2S
¢Ū2H2S+SO2=3S”ż+2H2O
¢ÜNa2SO3+S Na2S2O3
Na2S2O3
×Ü·“Ó¦ĪŖ£ŗ2Na2S+Na2CO3+4SO2= 3Na2S2O3+CO2
Ėę×ŶžŃõ»ÆĮņĘųĢåµÄĶØČė£¬æ“µ½ČÜŅŗÖŠÓŠ“óĮæĒ³»ĘÉ«¹ĢĢåĪö³ö£¬¼ĢŠųĶضžŃõ»ÆĮņĘųĢ壬·“Ó¦Ō¼°ėŠ”Ź±”£µ±ČÜŅŗÖŠpH½Ó½ü»ņ²»Š”ÓŚ7Ź±£¬¼“æÉĶ£Ö¹ĶØĘųŗĶ¼ÓČČ”£ČÜŅŗPHŅŖæŲÖĘ²»Š”ÓŚ7ĄķÓÉŹĒ
£ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©”£
¢ņ£®·ÖĄėNa2S2O3”¤5H2O²¢±ź¶ØČÜŅŗµÄÅØ¶Č£ŗ

£Ø1£©ĪŖ¼õÉŁ²śĘ·µÄĖšŹ§£¬²Ł×÷¢ŁĪŖ £¬²Ł×÷¢ŚŹĒ³éĀĖĻ“µÓøÉŌļ£¬ĘäÖŠĻ“µÓ²Ł×÷ŹĒÓĆ
£ØĢīŹŌ¼Į£©×÷Ļ“µÓ¼Į”£
£Ø2£©Õō·¢ÅØĖõĀĖŅŗÖ±ÖĮČÜŅŗ³ŹĪ¢»ĘÉ«»ė×ĒĪŖÖ¹£¬Õō·¢Ź±ĪŖŹ²Ć“ŅŖæŲÖĘĪĀ¶Č²»ŅĖ¹żøß
£Ø3£©³ĘČ”Ņ»¶ØÖŹĮæµÄ²śĘ·ÅäÖĆ³ÉĮņ“śĮņĖįÄĘČÜŅŗ£¬²¢ÓĆ¼ä½ÓµāĮæ·Ø±ź¶ØøĆČÜŅŗµÄÅØ¶Č£ŗÓĆ·ÖĪöĢģĘ½×¼
Č·³ĘČ”»ł×¼ĪļÖŹK2Cr2O7£ØĦ¶ūÖŹĮæ294g/mol£©0.5880æĖ”£Ę½¾ł·Ö³É3·Ż·Ö±š·ÅČė3øö׶ŠĪĘæÖŠ£¬¼Ó
Ė®Åä³ÉČÜŅŗ£¬²¢¼ÓČė¹żĮæµÄKI²¢Ėį»Æ£¬·¢ÉśĻĀĮŠ·“Ó¦£ŗ6I-+Cr2O72-+14H+=3I2+2Cr3++7H2O£¬ŌŁ¼ÓČė¼øµĪ
µķ·ŪČÜŅŗ£¬Į¢¼“ÓĆĖłÅäNa2S2O3ČÜŅŗµĪ¶Ø£¬·¢Éś·“Ó¦£ŗI2+2S2O32-=2I-+S4O62-£¬µĪ¶ØÖÕµćµÄĻÖĻóĪŖ
Čż“ĪĻūŗÄNa2S2O3ČÜŅŗµÄĘ½¾łĢå»żĪŖ20.00mL£¬ŌņĖł±ź¶ØµÄĮņ“śĮņĖįÄĘČÜŅŗ
µÄÅضČĪŖ mol/L”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğĢģ½ņŹŠøßČżµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©ĢžAŹĒŅ»ÖÖÖŲŅŖµÄ»ł±¾»Æ¹¤ŌĮĻ£¬ÓĆÖŹĘ׷زāµĆĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ28”£ĻĀĶ¼ŹĒŅŌAĪŖŌĮĻŗĻ³ÉŅ©ĪļÖŠ¼äĢåEŗĶŹ÷Ö¬KµÄĀ·Ļß”£
ŅŃÖŖ£ŗI. 
II. 
£ØR”¢R”ƱķŹ¾Ģž»ł»ņĒāŌ×Ó£©
£Ø1£©AÖŠ¹ŁÄÜĶŵĽį¹¹¼ņŹ½ŹĒ ”£ÓŠ»śĪļBµÄĆū³Ę
£Ø2£©B”śCµÄ»Æѧ·½³ĢŹ½ĪŖ ”£BŗĶĒāŃõ»ÆÄʵÄĖ®ČÜŅŗ¼ÓČČ·“Ó¦ĖłµĆµ½µÄÓŠ»ś²śĪļŗĶŅŅ¶žĖį·“Ӧɜ³Éøß·Ö×Ó»ÆŗĻĪļ£¬Š“³öÉś³Éøß·Ö×Ó»ÆŗĻĪļ·“Ó¦µÄ»Æѧ·½³ĢŹ½
£Ø3£©EµÄ·Ö×ÓŹ½ĪŖC4H8O”£ĻĀĮŠ¹ŲÓŚEµÄĖµ·ØÕżČ·µÄŹĒ £ØĢī×ÖÄøŠņŗÅ£©”£
a. ÄÜÓė½šŹōÄĘ·“Ó¦ b. ·Ö×ÓÖŠ4øöĢ¼Ō×ÓŅ»¶Ø¹²Ę½Ćę
c. Ņ»¶ØĢõ¼žĻĀ£¬ÄÜÓėÅØĒāäåĖį·“Ó¦ d. ÓėCH2=CHCH2OCH2CH3»„ĪŖĶ¬ĻµĪļ
£Ø4£©G”śHÉę¼°µ½µÄ·“Ó¦ĄąŠĶÓŠ ”£
£Ø5£©IµÄ·Ö×ÓŹ½ĪŖC4H6O2£¬Ęä½į¹¹¼ņŹ½ĪŖ ”£
£Ø6£©J”śKµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø7£©Š“³öÓėE¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄĖłÓŠĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ
£Ø²»æ¼ĀĒĖ³·“Ņģ¹¹£¬²»æ¼ĀĒ”ŖOHĮ¬ŌŚĖ«¼üĢ¼ÉĻµÄ½į¹¹£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĢžAŹĒŅ»ÖÖÖŲŅŖµÄ»ł±¾»Æ¹¤ŌĮĻ£¬ÓĆÖŹĘ׷زāµĆĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ28”£ĻĀĶ¼ŹĒŅŌAĪŖŌĮĻŗĻ³ÉŅ©ĪļÖŠ¼äĢåEŗĶŹ÷Ö¬KµÄĀ·Ļß”£

|
|
|
|
|
|
£ØR”¢R”ƱķŹ¾Ģž»ł»ņĒāŌ×Ó£©
£Ø1£©AÖŠ¹ŁÄÜĶŵĽį¹¹¼ņŹ½ŹĒ ”£
£Ø2£©B”śCµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©EµÄ·Ö×ÓŹ½ĪŖC4H8O”£ĻĀĮŠ¹ŲÓŚEµÄĖµ·ØÕżČ·µÄŹĒ £ØĢī×ÖÄøŠņŗÅ£©”£
a. ÄÜÓė½šŹōÄĘ·“Ó¦ b. ·Ö×ÓÖŠ4øöĢ¼Ō×ÓŅ»¶Ø¹²Ę½Ćę
c. Ņ»¶ØĢõ¼žĻĀ£¬ÄÜÓėÅØĒāäåĖį·“Ó¦ d. ÓėCH2=CHCH2OCH2CH3»„ĪŖĶ¬ĻµĪļ
£Ø4£©G”śHÉę¼°µ½µÄ·“Ó¦ĄąŠĶÓŠ ”£
£Ø5£©IµÄ·Ö×ÓŹ½ĪŖC4H6O2£¬Ęä½į¹¹¼ņŹ½ĪŖ ”£
£Ø6£©J”śKµÄ»Æѧ·½³ĢŹ½ĪŖ
ӣ
£Ø7£©Š“³öÓėE¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄĖłÓŠĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£Ø²»æ¼ĀĒĖ³·“Ņģ¹¹£¬²»æ¼ĀĒ”ŖOHĮ¬ŌŚĖ«¼üĢ¼ÉĻµÄ½į¹¹£©£ŗ
_______________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com