
| A���٢ڢ� | B���ڢۢ� | C���ܢݢ� | D���ڢۢ� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ԭ�Ӿ���ɱ��кܸߵ��ۡ��е㣬�кܴ��Ӳ�� |
| B��ԭ�Ӿ���ɱ�������������������� |
| C��ԭ�Ӿ���ɱ�Ӳ�ȴ�������ĥ���� |
| D��ÿĦԭ�Ӿ���ɱ��к�4mol C��O�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
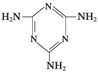 �׳ơ����������������������谷����������
�׳ơ����������������������谷����������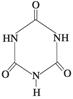 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

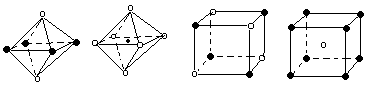
| A���� | B���� | C���� | D���� |
| ���ӻ����� | H2S | SnCl62- | PH3 | ClO4-- |
| �ռ乹�� | | | | |
�鿴�𰸺ͽ���>>
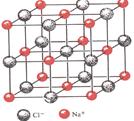
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
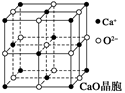
| A����NaCl�����У���Na�������Cl���γ��������� |
| B����NaCl�����У�ÿ������ƽ��ռ��4��Na�� |
| C����CsCl�����У�ÿ������ƽ��ռ��8��Cs�� |
| D��ͭ����Ϊ���������ѻ���ͭԭ�ӵ���λ��������һ��ͭԭ�����������ͭԭ�ӵĸ�����Ϊ12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͨ��״���£�60 g SiO2�����к��еķ�����ΪNA(NA��ʾ�����ӵ�����) |
| B��60 g SiO2����������2NA��Si��O�� |
| C����������ͬһ��ԭ��������4����ԭ�Ӵ���ͬһ�������4������ |
| D��SiO2�����к���1����ԭ�ӣ�2����ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��0.585/4a3�� | B��58.5/8a3�� | C��58.5/2a3�� | D��117/a3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A����Ͳ | B���ձ� | C������ƿ | D���Թ� |
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ʯ�����У�̼ԭ������C��C����֮��Ϊ1:2 |
B��720g C60���庬��NA����ͼ�о�����Ԫ |
| C�����Ӿ�����ÿ��������Χ��������6�����෴��ɵ����� |
| D��Cu�Ķѻ���ʽ�������������ܶѻ�����λ����8 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com