

| A��N2(g)+3H2(g)��2NH3(1)�� ��H��2(a-b-c)kJ��mol��1 |
| B��N2(g)+3H2(g)��2NH3(g)����H��2(b-a)kJ��mol��1 |
C�� N2(g)+ N2(g)+ H2(g)��NH3(1)����H��(b+c-a)kJ��mol��1 H2(g)��NH3(1)����H��(b+c-a)kJ��mol��1 |
D�� N2(g)+ N2(g)+ H2(g)��NH3(g)�� ��H��(a+b)kJ��mol H2(g)��NH3(g)�� ��H��(a+b)kJ��mol |


 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CH3OH��l��+ 3/2O2��g��=CO2��g��+ 2H2O��l������H="+725.8" kJ��mol��1 |
| B��2CH3OH��l��+ 3O2��g��= 2CO2��g��+ 4H2O��l������H="-1452" kJ��mol��1 |
| C��2CH3OH��l��+ 3O2��g��= 2CO2��g��+ 4H2O��l������H="-725.8" kJ��mol��1 |
| D��2CH3OH��l��+ 3O2��g��= 2CO2��g��+ 4H2O��l������H="+1452" kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
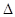 H=��67.7 kJ��mol-1
H=��67.7 kJ��mol-1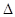 H=��57.3 kJ��mol-1
H=��57.3 kJ��mol-1A�������ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ��HF(aq) F-(aq)+H+(aq)�� F-(aq)+H+(aq)��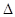 H=��10.4 kJ��mol-1 H=��10.4 kJ��mol-1 |
| B����V=20ʱ����Һ�У�c(OH-)=c(HF)+c(H+) |
| C����V=20ʱ����Һ�У�c(F-)��c(Na+)=0.1 mol��L-1 |
| D����V��0ʱ����Һ��һ�����ڣ�c(Na+)��c(F-)��c(OH-)��c(H+) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��2H+(aq) +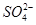 (aq)+ (aq)+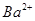 (aq)+2 (aq)+2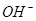 (aq) = BaSO4(s)+2H (aq) = BaSO4(s)+2H O(l); O(l);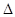 H = H =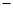 57.3 kJ/mol 57.3 kJ/mol |
B��KOH(aq)+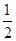 H H SO4(aq) = SO4(aq) = 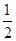 K K SO4(aq)+H SO4(aq)+H O(l); O(l); H= H= 57.3kJ/mol 57.3kJ/mol |
C��C8H18(l)+ 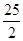 O O (g)=8CO (g)=8CO (g)+ 9H (g)+ 9H O(g); O(g);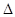 H= H=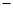 5518 kJ/mol 5518 kJ/mol |
D��2C8H18(g)+25O (g)=16CO (g)=16CO (g)+18H (g)+18H O(1); O(1);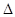 H= H=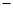 5518 kJ/mol 5518 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2HI(g)�� ��H����9.48 kJ/mol��
2HI(g)�� ��H����9.48 kJ/mol�� 2HI(g)�� ��H����26.48 kJ/mol
2HI(g)�� ��H����26.48 kJ/mol| A��1 mol I2(g)��ͨ��1 mol H2(g)����Ӧ����9.48 kJ |
| B��1 mol��̬����1 mol��̬���������������17.00 kJ |
| C���ֽ�1 mol HI (g)����H2(g)��̬��ķ�Ӧ����13.24kJ |
| D����Ӧ(��)�ķ�Ӧ���������ȷ�Ӧ(��)�ķ�Ӧ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
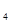 +5O
+5O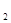 =P
=P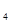 O
O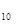 ����֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ��P��Pa kJ��mol
����֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ��P��Pa kJ��mol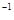 ��P��O b kJ��mol
��P��O b kJ��mol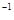 ��P="=O" c kJ��mol
��P="=O" c kJ��mol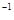 ��O="=O" d kJ��mol
��O="=O" d kJ��mol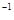
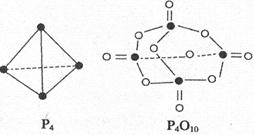
A����6a+5d��4c��12b�� kJ��mol | B����4c+12b��6a��5d�� kJ��mol |
C����4c+12b��4a��5d�� kJ��mol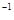 | D����4a+5d��4c��12b�� kJ��mol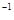 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������88kJ | B������2.44KJ | C������44kJ | D������44KJ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com