
ʵ������һƿ�����Ȼ��Ƶ��������ƹ����Լ������ⶨNaOH����������ԼΪ82.0%��Ϊ����֤�䴿�ȣ���Ũ��Ϊ0.2mol/L��������еζ����Իش��������⣺
(1) ������ƽ����5.0g�����Լ���������ˮ�ܽ����ձ��У�����Ȼ������ֱ��ת�� 500mL����ƿ�У�ǡ�����̶��ߣ���ɴ���Һ���á���������ϲ����г��ֵ��崦����
��____________________��__________________��____________________________
��____________________��___________________��
(2)��������װ��25.00mL �ζ����У�����Һ��λ���� ���������¿̶ȡ�
(3)ȡ20.00mL����Һ�����ⶨ������ʵ���������Ҫ���� �С��� �Լ���ָʾ��ʱ���ζ�����Һ��ɫ�� �պ��� ɫʱΪֹ��
(4)�ζ����յ����������ȥ20.00mL������NaOH����������Ϊ ��
(5)�Է����ζ���������������Щʵ���������� ��
A��ת�ƴ���Һ������ƿʱ��ĩϴ���ձ�
B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ����
C���ζ�ʱ����Ӧ����ҡ��̫���ң�������Һ�彦��
D���ζ����յ�ʱ���ζ��ܼ�������Һ��
E�����ζ��ܿ�ʼʱ���ӣ����յ�ʱ����
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
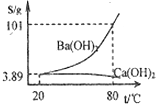
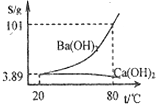 ������ʵ��������ʵ����ʵ����������ȷ����
������ʵ��������ʵ����ʵ����������ȷ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ��Ŀ�� | ���鷽�� |
| �����Ȼ������Ƿ���� | D D |
| ��ȥʳ��������ϸɳ | C C |
| ��ȥ̼���ƹ���������̼������ | A A |
| ��ȥþ���л��е��������� | B B |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ���۲쵽��ƿ����Һ��ڣ�װ�ã����о������ữ�ĸ��������Һ��ɫ��
��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ���۲쵽��ƿ����Һ��ڣ�װ�ã����о������ữ�ĸ��������Һ��ɫ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ƺ��ص�һ����ѧ��һ��ѧ�ڵ������¿���ѧ������������ ���ͣ�ʵ����
��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ: CH3CH2OH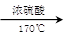 CH2=CH2��+H2O������һ��ʱ�����Һ���к�ɫ������֡���һ��ʱ��������ữ�ĸ��������Һ��ɫ����������֪�������������к���CH2=CH2��SO2��CO2��H2O��
CH2=CH2��+H2O������һ��ʱ�����Һ���к�ɫ������֡���һ��ʱ��������ữ�ĸ��������Һ��ɫ����������֪�������������к���CH2=CH2��SO2��CO2��H2O��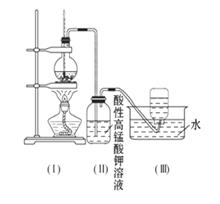
���Ը��������Һ��ɫ����ͬѧ��Ϊ��֤����ϩ�����Ը��������Һ�����ˣ���ͬѧ��Ϊ����֤����ϩ�����Ը��������Һ�����ˡ�
��1������Ϊ�ĸ�ͬѧ�Ĺ۵���ȷ�� _____ ����ס����ҡ����������ǣ�������ѡ����ѡ��_____
| A��(��)ƿ�����Ը��������Һ��ɫ����֤����ϩ������������Ӧ |
| B��(��)ƿ�����Ը��������Һ��ɫ����֤����ϩ�����˼ӳɷ�Ӧ |
| C��(��ƿ�����Ը��������Һ��ɫ������֤��ͨ��������Ǵ����� |
| D��(��)ƿ�����Ը��������Һ��ɫ��ֻ��֤��ͨ�������һ�����л�ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������ʵ��������ʵ����ʵ����������ȷ����______ ������ţ���
������ʵ��������ʵ����ʵ����������ȷ����______ ������ţ����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com