
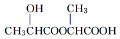 ����������W��Ӧ�γɻ�����д����Ӧ����ʽ��
����������W��Ӧ�γɻ�����д����Ӧ����ʽ��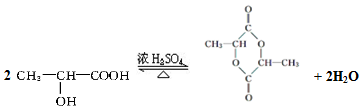 W�����γɸ߷��ӻ�����-�������ṹ��ʽ�ɱ�ʾΪ
W�����γɸ߷��ӻ�����-�������ṹ��ʽ�ɱ�ʾΪ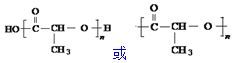 ��
��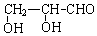 ����֪ͬһ��̼ԭ���ϲ�������2���ǻ�����
����֪ͬһ��̼ԭ���ϲ�������2���ǻ�����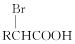
���� W����NaHCO3��Ӧ�������к���-COOH��W�ķ���ʽΪC3H6O3�������Ͷ�Ϊ$\frac{2��3+2-6}{2}$=1�������к������ֻ�Բ�ͬ����ԭ�ӣ�������Ϊ3��1��1��1�������к���1��-CH3��-COOH����Ϸ���ʽ��֪������-OH��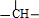 ����WΪCH3CH��OH��COOH��A��B��C��D��W�����к�����̼ͬԭ��������Ϸ�Ӧ��Ϣ����W�Ľṹ��֪��DΪCH3CHBrCOOH��CΪCH3CH2COOH����AΪCH3CH2CH2OH��BΪCH3CH2CHO���Դ˽����⣻
����WΪCH3CH��OH��COOH��A��B��C��D��W�����к�����̼ͬԭ��������Ϸ�Ӧ��Ϣ����W�Ľṹ��֪��DΪCH3CHBrCOOH��CΪCH3CH2COOH����AΪCH3CH2CH2OH��BΪCH3CH2CHO���Դ˽����⣻
��� �⣺��1�������Ϸ�����֪WΪCH3CH��OH��COOH���ʴ�Ϊ��CH3CH��OH��COOH��
��2��CH3CH��OH��COOH��һ�������£��ж�����ˮ��ʽ����ͨ�����Ӽ��������ɣ���ͨ���������������ɣ���������W��Ӧ�γ���״�����ṹ��ʽΪ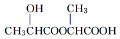 ����������W��Ӧ�γɻ�����д����Ӧ����ʽΪ
����������W��Ӧ�γɻ�����д����Ӧ����ʽΪ ��W�����γɸ߷��ӻ�����-�������ṹ��ʽ�ɱ�ʾ
��W�����γɸ߷��ӻ�����-�������ṹ��ʽ�ɱ�ʾ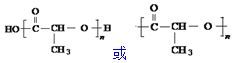 ��
��
�ʴ�Ϊ��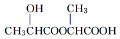 ��
�� ��
��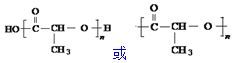 ��
��
��3��W��ij��ͬ���칹������������ʣ��ܷ���������Ӧ��˵������ȩ����1mol�������ܸ������Ʒ�Ӧ����1molH2��˵������2���ǻ������ͬ���칹��Ľṹ��ʽΪ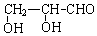 ���ʴ�Ϊ��
���ʴ�Ϊ��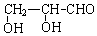 ��
��
��4����AΪCH3CH2CH2OH������Ϊ1һ�������ʴ�Ϊ��1һ������
��BΪCH3CH2CHO��������Cu��OH��2��Ӧ�Ļ�ѧ����ʽ��CH3CH2CHO+2Cu��OH��2$\stackrel{����}{��}$CH3CH2COOH+Cu2O��+2H2O��
�ʴ�Ϊ��CH3CH2CHO+2Cu��OH��2$\stackrel{����}{��}$CH3CH2COOH+Cu2O��+2H2O��
��ij�л�����C��Ϊͬ���칹�壬���������࣬�ֺ�ȩ����ӦΪ�������Ҵ��γɵ������ṹ��ʽΪHCOOC2H5���ʴ�Ϊ��HCOOC2H5��
���� ���⿼���л�����ƶ���ϳɣ�Ϊ��Ƶ���㣬���ؿ���ѧ���ķ�����������Ҫѧ���Ը������Ϣ�������ã����չ����ŵ��ݱ��ǹؼ����ѶȲ���ע�����֪ʶ���������գ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
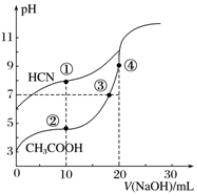
| A�� | ���ʱ��c��CN-����c��Na+����c��HCN����c��OH-�� | |
| B�� | ���ʱ��c��Na+��=c��CH3COO-����c��H+�� | |
| C�� | ���ʱ��c��Na+����c��CH3COO-����c��OH-����c��H+�� | |
| D�� | ��ٺ͵����ʾ��Һ�У�c��CH3COO-��-c��CN-��=c��HCN��-c��CH3COOH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
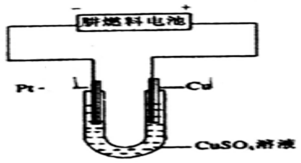 �ش��������⣺
�ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����� | ʵ������ | ���� |
| A | ����ľƾ���Һ����һ��������ˮ�е���ɫ��Һ�壬�ü�������� | �ж����ЧӦ | ��ɫ��Һ��Ϊ���� |
| B | ��FeSO4����ǿ�ȣ��ú�ɫ���壬����������ͨ��BaCl2��Һ | ������ɫ���� | ��ɫ����ΪBaSO4��BaSO3 |
| C | ȡ������X�ֱ�ͨ��Ʒ����Һ�����Ը��������Һ | ����Һ����ɫ | X��������ϩ |
| D | ����ҺY�еμ����ᣬ�ٵμ�BaCl2��Һ | �а�ɫ���� | Y��һ������SO42- |
| A�� | A�� | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
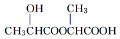 ��
�� ��
��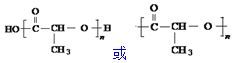 ��
��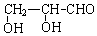 ����֪ͬһ��̼ԭ���ϲ�������2���ǻ�����
����֪ͬһ��̼ԭ���ϲ�������2���ǻ�����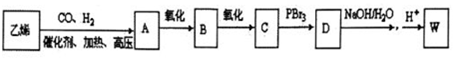

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����Ƿ������绹�ǻ������磬���ǽ���ѧ��ת��Ϊ���� | |
| B�� | PM2.5���е�Ǧ���ӡ�����������ȶ������к���Ԫ�ؾ��ǽ���Ԫ�� | |
| C�� | ���������͵������������γ��������Ҫ���� | |
| D�� | ������ϴ�Ӽ������ƹ�ʹ�ã�������Ч����ˮ�帻Ӫ�����ķ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ҵ���ˮ | B�� | �������������� | C�� | ��������Ҵ� | D�� | ���ȼ����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
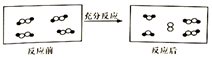 һ�������£�ij�����и����ڷ�Ӧǰ��仯��ʾ��ͼ���£����С�͡������ͬԪ�ص�ԭ�ӣ����ڴ˷�Ӧ˵��������ǣ�������
һ�������£�ij�����и����ڷ�Ӧǰ��仯��ʾ��ͼ���£����С�͡������ͬԪ�ص�ԭ�ӣ����ڴ˷�Ӧ˵��������ǣ�������| A�� | һ�����ڿ��淴Ӧ | B�� | һ�����ڷ��ȷ�Ӧ | ||
| C�� | һ������������ԭ��Ӧ | D�� | һ�����ڷֽⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �� | H2C2O4 | HCN | H2CO3 |
| ���볣����25�棩 | K1=5.4��10-2 K2=5.4��10-5 | 4.9��10-10 | K1=4.4��10-7 K2=4.7��10-11 |
| A�� | NaCN+H2O+CO2��������=HCN+NaHCO3 | |
| B�� | 0.1mol•L-1��Na2CO3��Һ�μӵ�ͬŨ�ȵ�HCN��Һ�У�������˵��δ������Ӧ | |
| C�� | Ũ�Ⱦ�Ϊ0.1mol•L-1��Na2CO3��NaHCO3��NaCN��NaHC2O4��Һ��pH������NaCN | |
| D�� | NaHC2O4��Һ�У�����Ũ���ɴ�С��˳���� c��Na+ ����c��H+ ����c��HC2O4- ����c��C2O42- ����c��OH- �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com