
£Ø1£©2012ÄźĀ׶Ų°ĀŌĖ»į»š¾ę²ÉÓƱūĶéĪŖČ¼ĮĻ”£±ūĶéČČÖµ½Ļøߣ¬ĪŪČ¾½ĻŠ”£¬ŹĒŅ»ÖÖÓÅĮ¼µÄČ¼ĮĻ”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ

¢ŁČēĶ¼ŹĒŅ»¶ØĮæ±ūĶéĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶ1 mol H2O(l)¹ż³ĢÖŠµÄÄÜĮæ±ä»ÆĶ¼£¬ĒėŌŚĶ¼ÖŠµÄĄØŗÅÄŚĢīČė”°£«”±»ņ”°£”±”£
¢ŚŠ“³ö±ķŹ¾±ūĶéČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½£ŗ___________________________________”£
¢Ū¶ž¼×ĆŃ(CH3OCH3)ŹĒŅ»ÖÖŠĀŠĶČ¼ĮĻ£¬Ó¦ÓĆĒ°¾°¹ćĄ«”£1 mol¶ž¼×ĆŃĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬Ė®·Å³ö1 455 kJČČĮ攣Čō1 mol±ūĶéŗĶ¶ž¼×ĆѵĻģŗĻĘųĢåĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬Ė®¹²·Å³ö1 645 kJČČĮ棬Ōņ»ģŗĻĘųĢåÖŠ£¬±ūĶéŗĶ¶ž¼×ĆѵÄĪļÖŹµÄĮæÖ®±ČĪŖ________”£
£Ø2£©øĒĖ¹¶ØĀÉČĻĪŖ£ŗ²»¹Ü»Æѧ¹ż³ĢŹĒŅ»²½Ķź³É»ņ·Ö¼ø²½Ķź³É£¬Õūøö¹ż³ĢµÄ×ÜČČŠ§Ó¦ĻąĶ¬”£ŹŌŌĖÓĆøĒĖ¹¶ØĀÉ»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁŅŃÖŖ£ŗH2O(g)=H2O(l)””¦¤H1£½£Q1 kJ/mol C2H5OH(g)=C2H5OH(l)””¦¤H£½£Q2 kJ/mol
C2H5OH(g)£«3O2(g)=2CO2(g)£«3H2O(g)””¦¤H3£½£Q3 kJ/mol
ČōŹ¹46 gŅŗĢ¬ĪŽĖ®¾Ę¾«ĶźČ«Č¼ÉÕ£¬²¢»Öø“µ½ŹŅĪĀ£¬ŌņÕūøö¹ż³ĢÖŠ·Å³öµÄČČĮæĪŖ________kJ”£
¢ŚĢ¼(s)ŌŚŃõĘų¹©Ó¦²»³ä×揱£¬Éś³ÉCOĶ¬Ź±»¹²æ·ÖÉś³ÉCO2£¬Ņņ“ĖĪŽ·ØĶعżŹµŃéÖ±½Ó²āµĆ·“Ó¦£ŗ C(s)£« O2(g)=CO(g)µÄ¦¤H”£µ«æÉÉč¼ĘŹµŃ锢ĄūÓĆøĒĖ¹¶ØĀɼĘĖć³öøĆ·“Ó¦µÄ¦¤H£¬¼ĘĖ揱ŠčŅŖ²āµĆµÄŹµŃ鏿¾ŻÓŠ________”£
O2(g)=CO(g)µÄ¦¤H”£µ«æÉÉč¼ĘŹµŃ锢ĄūÓĆøĒĖ¹¶ØĀɼĘĖć³öøĆ·“Ó¦µÄ¦¤H£¬¼ĘĖ揱ŠčŅŖ²āµĆµÄŹµŃ鏿¾ŻÓŠ________”£
£Ø1£©¢Ł£””¢ŚC3H8(g)£«5O2(g)=3CO2(g)£«4H2O(l)””¦¤H£½£2 215.0 kJ/mol””¢Ū1”Ć3
£Ø2£©¢Ł3Q1£Q2£«Q3””¢ŚĢ¼ŗĶCOµÄČ¼ÉÕČČ
”¾½āĪö”æ£Ø1£©¢Ł±ūĶéĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶ1 mol H2O(l)µÄ¹ż³Ģ·ÅČČ£¬¦¤HĪŖ”°£”±”£
¢ŚČ¼ÉÕČČŹĒ½«1 molĪļÖŹĶźČ«Č¼ÉÕÉś³ÉĪȶØŃõ»ÆĪļŹ±·Å³öµÄČČĮ棬ĖłŅŌ±ķŹ¾±ūĶéČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ĪŖC3H8(g)£«5O2(g)=3CO2(g)£«4H2O(l) ¦¤H£½£2 215.0 kJ/mol”£
¢Ūn(¶ž¼×ĆŃ)”Į1 455 kJ/mol£«[1 mol£n(¶ž¼×ĆŃ)]”Į2 215.0 kJ/mol£½1 645 kJ£¬½āµĆn(¶ž¼×ĆŃ)£½0.75 mol£¬n(±ūĶé)£½0.25 mol”£
£Ø2£©¢ŁÓÉ¢Ł”Į¢Ū£«¢Ū£¢ŚæɵĆC2H5OH(l)£«3O2(g)=2CO2(g)£«3H2O(l)””¦¤H4£½£(3Q1£Q2£«Q3) kJ/mol£¬ĖłŅŌŹ¹46 gŅŗĢ¬ĪŽĖ®¾Ę¾«ĶźČ«Č¼ÉÕ£¬²¢»Öø“µ½ŹŅĪĀ£¬Õūøö¹ż³ĢÖŠ·Å³öµÄČČĮæĪŖ(3Q1£Q2£«Q3) kJ”£
¢ŚĄūÓĆøĒĖ¹¶ØĀɼĘĖć·“Ó¦C(s)£«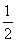 O2(g)=CO(g)µÄ¦¤H£¬ŠčŅŖ²āµĆµÄŹµŃ鏿¾ŻÓŠĢ¼ŗĶCOµÄČ¼ÉÕČČ”£
O2(g)=CO(g)µÄ¦¤H£¬ŠčŅŖ²āµĆµÄŹµŃ鏿¾ŻÓŠĢ¼ŗĶCOµÄČ¼ÉÕČČ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com