
[ ]
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ȷ�-CH2Cl2 | B��ʯ��-2CaSO4?H2O | C��Ư��-Ca��ClO��2��CaCl2�Ļ���� | D��â��-Na2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
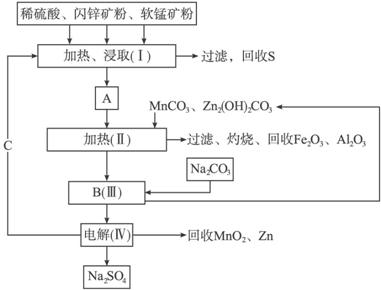
��֪��A��MnSO4��ZnSO4��Fe2(SO4)3��Al2(SO4)3�Ļ��Һ��
�ڢ��еĵ�ⷴӦʽΪMnSO4+ZnSO4+2H2O![]() MnO2+ Zn+2H2SO4��
MnO2+ Zn+2H2SO4��
(1)A�����ڻ�ԭ�������__________��
(2)MnCO3��Zn2(OH)2CO3��������____________________________________������Ҫ���ȵ�ԭ����____________________________________��C�Ļ�ѧʽ��____________��
(3)�������г��õ�MnO2��Zn���⣬���ɵõ��ĸ���Ʒ��____________��
(4)��������������е���ģ�����ʯ�⣬�蹺��Ļ���ԭ����____________��
(5)Ҫ��Na2SO4��Һ�еõ�â��(Na2SO4��10H2O)������еIJ���������Ũ����____________�����ˡ�ϴ�ӡ�����ȡ�
(6)������MnO2��Zn�ĽǶȼ��㣬���̿����п��������ȴ�Լ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ͻ�ѧʽ���ϵ��ǣ� ��
�ٴ���NaCl �ڿ�����Na ���մ�NaHCO3 ���ռ� NaOH��â��Na2SO4��10H2O
A.�٢� B.�ۢ� C.�ڢ� D.�ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�������׳��뻯ѧʽ����Ӧ����
A���̷�-FeSO4��7H2O B��â��-Na2SO4��10H2O
C������- Al2(SO4)3��12H2O D������- CuSO4��5H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���Ϻ���������������ѧ����ĩ��ѧ������⻯ѧ�Ծ��������棩 ���ͣ������
ij��ȤС��̽����â��Na2SO4��10H2O��CaOΪԭ���Ʊ�Na2CO3��
��1����CaOˮ������â���γ�Na2SO4��Ca(OH)2��H2O��Ԫ��ϵ����Ӧ����ˣ�����Һ��ͨ��CO2�������õ�Na2CO3����Ԫ��ϵ�з�Ӧ�����ӷ���ʽΪ�� SO42��+ Ca(OH)2(s)+2H2O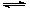 CaSO4��2H2O(s)+2 OH��
CaSO4��2H2O(s)+2 OH��
�÷�Ӧ��ƽ�ⳣ������ʽK=_________________________��
��Na2SO4��Ca(OH)2��H2O��Ԫ��ϵ������������ij���������ʣ�����pH=12.3 [��c(OH��)=0.02mol/L]����ʹ��Ӧ�ڳ����������С���Ӧ����ˣ�������Һ��ͨ��CO2����һ�������õ�Na2CO3��
��2����Na2SO4��Ca(OH)2��H2O��Ԫ��ϵ�в�ֱ��ͨ��CO2����������_______________________________________________________________��
��3�����ӵ����������������������д�����㣩��_____________��______________��
��4����ƽ���ƶ�ԭ�����������������ʵ����ɣ�____________________________________����HA��ʾ�����ӵ����ʣ����ܷ�Ӧ�����ӷ���ʽ��дΪ_______________________��
��5��Na2CO3��Һ�д���ˮ��ƽ�⣺CO32����H2O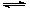 HCO3����OH��������˵���������_________��
HCO3����OH��������˵���������_________��
a����ˮϡ�ͣ���Һ���������ӵ�Ũ�ȶ���С
b��ͨ��CO2����ҺpH��С
c������NaOH���壬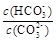 ��С
��С
d��ϡ����Һ��ƽ�ⳣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com