
��ͼij��ѧʵ��С����̵���ص�Ũ�����Լ���ǩ�IJ������ݣ���С���ڿ��������л��������Ϣ��Ũ���ḯʴ�Ժ�ǿ������ˮʱ�ͷŴ������ȣ�Ũ���������ֲ����롣
|
��HSO4- ![]() SO42- + H+
SO42- + H+
�����Լ�ƿ���ܷ�ǩ������,���ǻ��ɸ����������������ǩ����,ȷ����չ�о�.
����Ϊ������һ�־���������������Һ��C(H+),��C(H+) =36.8 mol•L-1,����Һ����������Ϊ0.98;
����Ϊ����ʹ�о��ܵ�����������Ҳ���У��������������������⡣�������룺ȡһ����������������BaCl2��Ӧ�����ˡ�ϴ�ӡ������������������������
�������á��к͵ζ������в������������£�A��ȷ��ȡһ����������ᣬ����������ˮϡ�ͣ�B����ϡ�ͺ���Һ�е���2-3�η�̪��C����һ��Ũ�ȵ�NaOH��Һ�ζ���ֱ�����ֵζ��յ�Ϊֹ��D����¼���ĵ�NaOH��Һ�������
�ش��������⣺��
��1�����ƶϡ���ͬѧ���������С��������ǣ� ��
��2���ҷ����Ĺؼ����������㣺��ȷ��SO42-��ȫ��Ӧ����ϴ�ӳ�����ȷ�������������ʡ���ʵ��������϶�SO42-��ȫ��Ӧ��
��
��3�����ϡ��Ũ������Һ��
��
��4�����������в�����ʹ�ⶨ���ƫ�� ��
���ڵζ�ǰδ��NaOH����Һ��ϴ�ζ���
�ڵζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���ζ��ܼ��첿��������ʧ
����ƿ��ˮϴ�ɾ���δ�ô���Һ��ϴ
�ܿ�ʼʱƽ�Ӷ������ζ����ʱ���Ӷ���
��5������ʵ���У��ֱ�ȡ����5.00mL,��5.00mol/L NaOH�ζ�����,�յ�ʱ,�õ���NaOH���������Ϊ35.65mL��40.62mL��35.55mL,��ͨ������������Ƿ�ϸ�.��Ҫ������̣�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ѧʵ��С��̽������ʳ�ð״��д����ȷŨ�ȣ�ȡ25.00 mLijƷ��ʳ�ð״�����ƿ�У���ʵ������Ũ��Ϊcb mol/L�ı�NaOH��Һ������еζ���
ij��ѧʵ��С��̽������ʳ�ð״��д����ȷŨ�ȣ�ȡ25.00 mLijƷ��ʳ�ð״�����ƿ�У���ʵ������Ũ��Ϊcb mol/L�ı�NaOH��Һ������еζ���| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
| (23.35+25.30)c |
| 2V |
| (23.35+25.30)c |
| 2V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ������ȣ���ȵ�λ��g/100ml�����ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��
ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ������ȣ���ȵ�λ��g/100ml�����ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ ��PH�� |
5.0-8.0 | 3.1-4.4 | 4.4-6.2 | 8.2-10.0 |
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/ml | 26.02 | 25.32 | 25.28 |
| 151.8C |
| V |
| 151.8C |
| V |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��
ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ��PH�� | 5.0��8.0 | 3.1��4.4 | 4.4��6.2 | 8.2��10.0 |
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/ml | 26.02 | 25.35 | 25.30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����
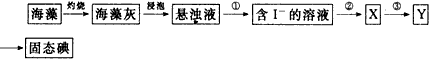
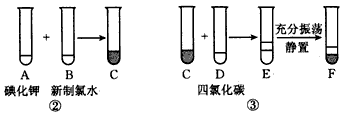
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com