
��10�֣������Ȼ�п����Ҫԭ����п���ȣ���Щ���Ϻ�п30%-70%�����к���������Fe��Cu�������Σ�����������������ͼ��ʾ����һ�ξ���������pH�Ĺ��̣���www.ks5.u.com
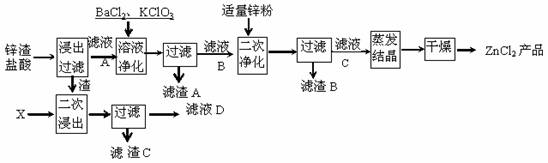
��1�����ν���ʱ�����X�Ļ�ѧʽ��______________����ҺDӦ�ú���ҺA��B��C�е���Һ �ϲ���
��2����һ�ξ���ʱ����BaCl2�������� ��
��3�����ξ�������˵õ�������B����Ҫ�ɷ��� ��
��4���������ӷ���ʽ��ʾ��������ص����� ��
��5����֪SOCl2��Һ̬������е���77�棬��ʢ��10mLˮ����ƿ��С�ĵμӼ���SOCl2���ɹ۲쵽��Ӧ���ң�Һ�����а����γɣ����ݳ��̼�����ζ�����塣��ҵ�ϳ���ZnCl2?3H2O��SOCl2��Ϲ�������ȡ��ˮ�Ȼ�п�������Ʒ������Ե�ԭ����
 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com