
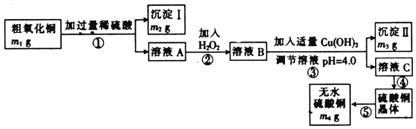
为测定粗氧化铜(其中含少量氧化亚铁及不溶于酸的杂质)中CuO的质量分数,并制取无水硫酸铜,某化学活动小组进行了下列实验:

已知Fe3+、Cu2+、Fe2+三种离子在溶液中形成氢氧化物沉淀的pH范围如下:
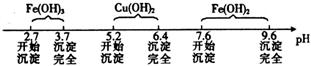
请回答下列问题:
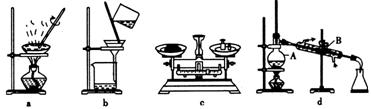
(1)在整个实验过程中,下列实验装置不可能用到的是 (填字母),装置d中仪器A和B的名称分别为 。
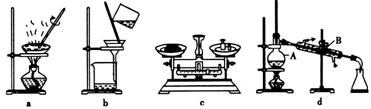
(2)溶液A中所含溶质的化学式为 。
(3)步骤②发生反应的离子方程式为 。
(4)沉淀II的化学式为 。
(5)粗氧化铜样品中CuO的质量分数为 。(列出计算式)
科目:高中化学 来源: 题型:



m1-m2-
| ||
| m1 |
m1-m2-
| ||
| m1 |
查看答案和解析>>
科目:高中化学 来源: 题型:
为测定粗氧化铜(其中含少量氧化亚铁及不溶于酸的杂质)中CuO的质量分数,并制取无水硫酸铜,某化学活动小组进行了下列实验:
已知Fe3+、Cu2+、Fe2+三种离子在溶液中形成氢氧化物沉淀的pH范围如下:
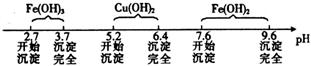
请回答下列问题:
(1)在整个实验过程中,下列实验装置不可能用到的是 (填字母),装置d中仪器A和B的名称分别为 。
(2)溶液A中所含溶质的化学式为 。
(3)步骤②发生反应的离子方程式为 。
(4)沉淀II的化学式为 。
(5)粗氧化铜样品中CuO的质量分数为 。(列出计算式)
查看答案和解析>>
科目:高中化学 来源: 题型:
为测定粗氧化铜(其中含少量氧化亚铁及不溶于酸的杂质)中CuO的质量分数,并制取无水硫酸铜,某化学活动小组进行了下列实验:
已知Fe3+、Cu2+、Fe2+三种离子在溶液中形成氢氧化物沉淀的pH范围如下:
请回答下列问题:
(1)在整个实验过程中,下列实验装置不可能用到的是 (填字母),装置d中仪器A和B的名称分别为 。
(2)溶液A中所含溶质的化学式为 。
(3)步骤②发生反应的离子方程式为 。
(4)沉淀II的化学式为 。
(5)粗氧化铜样品中CuO的质量分数为 。(列出计算式)
查看答案和解析>>
科目:高中化学 来源:2011届河北省唐山一中高三下学期第一次调研考试(理综)化学部分 题型:实验题
为测定粗氧化铜(其中含少量氧化亚铁及不溶于酸的杂质)中CuO的质量分数,并 制取无水硫酸铜,某化学活动小组进行了下列实验:
制取无水硫酸铜,某化学活动小组进行了下列实验:
已知Fe3+、Cu2+、Fe2+三种离子在溶液中形成氢氧化物沉淀的pH范围如下:
请回答下列问题:
(1)在整个实验过程中,下列实验装置不可能用到的是 (填字母),装置d中仪器A和B的名称分别为 。
(2)溶液A中所含溶质的化学式为 。
(3)步骤②发生反应的离子方程式为 。
(4)沉淀II的化学式为 。
(5)粗氧化铜样品中CuO的质量分数为 。(列出计算式)
查看答案和解析>>
科目:高中化学 来源:2010-2011学年河北省高三下学期第一次调研考试(理综)化学部分 题型:实验题
为测定粗氧化铜(其中含少量氧化亚铁及不溶于酸的杂质)中CuO的质量分数,并制取无水硫酸铜,某化学活动小组进行了下列实验:

已知Fe3+、Cu2+、Fe2+三种离子在溶液中形成氢氧化物沉淀的pH范围如下:

请回答下列问题:
(1)在整个实验过程中,下列实验装置不可能用到的是 (填字母),装置d中仪器A和B的名称分别为 。

(2)溶液A中所含溶质的化学式为 。
(3)步骤②发生反应的离子方程式为 。
(4)沉淀II的化学式为 。
(5)粗氧化铜样品中CuO的质量分数为 。(列出计算式)
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com