
��֪ϩ����Ȳ���ڳ����������·������·�Ӧ��
CH3-CH=CH-CH2-CH=CH2  CH3CHO+OHC-CH2-CHO+HCHO
CH3CHO+OHC-CH2-CHO+HCHO
CH3-C C-CH2-C
C-CH2-C CH
CH  CH3COOH+HOOC-CH2-COOH+HCOOH
CH3COOH+HOOC-CH2-COOH+HCOOH
ij������ʽΪC10H10���ڳ��������·�����Ӧ��
C10H10  CH3COOH+3HOOC-CHO+CH3CHO
CH3COOH+3HOOC-CHO+CH3CHO
(1) C10H10�����к��� ��˫���� ��������
(2) C10H10���ӽṹ��ʽΪ ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2L�ܱ������У����ֺ��£�����KClO3��MnO2����������ȡO2��5min������
O232�ˣ���˷�Ӧ��ƽ�����ʿɱ�ʾΪ�� ��
A . v(O2)��3.2g��L��1��min��1 B. v(O2)��0.1 mol��L��1��min��1
C. v(KCl)��0.1 mol��L��1��min��1 D. v(KClO3)��0.0667 mol��L��1��min��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȡ����Fe2O3��ĩ(����ɫ)�����������ᣬ������Ӧ�Ļ�ѧ����ʽ��___________________________________________________��
��Ӧ��õ�����Һ��________ɫ���ô���Һ�ֱ�������ʵ�飺
(1)ȡ������Һ�����Թ��У����뼸��NaOH��Һ���ɹ۲쵽�к��ɫ�������ɣ���Ӧ�Ļ�ѧ����ʽΪ_____________________________________________
________________________________________________________________���˷�Ӧ����________(�Ӧ����)��
(2)��С�ձ��м���20 mL����ˮ�����������ں����ˮ�е��뼸�α���FeCl3��Һ�������������Һ��________ɫ�����Ƶ�Fe(OH)3���塣
(3)ȡ��һֻС�ձ�Ҳ����20 mL����ˮ�����ձ��м���1 mL FeCl3��Һ�����Ⱥ����ձ�(��ż�)��ʢ��Fe(OH)3������ձ�(�����)һ������ڰ������ֱ��ü���������ձ��е�Һ�壬���Կ���________�ձ��е�Һ����������ЧӦ�����ʵ�������������_______________________________��
(4)��Fe(OH)3�����������ʵ�飺
�ٽ���װ��U�ι��У���ʯī�缫��ֱͨ���磬ͨ��һ��ʱ�����������������ɫ�����˵��____________________________________________
_________________________________________________________________�����������Ϊ________��
�������м��뱥��(NH4)2SO4��Һ������������________________��ԭ����________________ _____________________________________________
_____________________________________________ ______________________________________________________________________��
______________________________________________________________________��
�������е������ϡ���ᣬ������________________����ԭ����___________ _________________________________________________________________��
���ᴿ�˷�ɢϵ���õķ�����______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����һ±�����ڴ��������·�����Ӧ���ɱ���ͬϵ�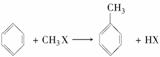 ���ڴ��������£��ɱ������и������ʺϳ��ұ����ѡ�õ���
���ڴ��������£��ɱ������и������ʺϳ��ұ����ѡ�õ���
A��CH3��CH3��Cl2 B��CH2===CH2��Cl2
C��CH2===CH2��HCl D��CH3��CH3��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ǡ����ȫ��Ӧ�����и����з�Ӧǰ�ֲ������
�ٵ������� ��ԭ������ �۷������� �����ʵ������� �����ʵ�������
A.�٢ڢ� B. �٢ۢ� C.�ڢۢ� D.�ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ������������������ϵ������ӡȾ��ýȾ����Ⱦ�ϻ�ԭ������������ұ��ҽҩ���������ҵ��һʵ��С��ģ�¹�ҵ������ȡ�Ȼ����������װ������
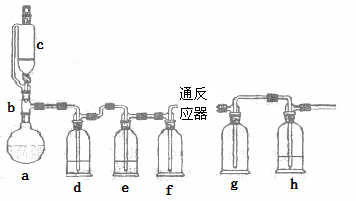

ͨ������������Ͽ�֪��
���ڳ�������500��ʱ�����봿��������Cl2��Ӧ������FeCl2�����¶Ƚϵ�ʱ������FeCl3��
��FeCl3�۷е�ͣ���������
����������Ϣ�ش���ص�����
��1��abc������ϳ���ȡ������װ�ã���Ӧ������ȣ���д��a�������������Ļ�ѧ��Ӧ����ʽ ��
��2��d��eʢװ��ҩƷ�ֱ��� �� ��f��g�������� ��
��3����Ӧ��ΪӲ�ʲ����ܣ�����������������500�����ҷ�Ӧ��
�ٻ�ѧ��Ӧ����ʽΪ
��ʵ�����˳���ǣ���װ������ ��װ��ҩƷ�� ��
��ֹͣ���ȡ��ر�c�Ļ�����
��4��ʵ��С���¼��ʵ���������£�
| �۲쵽�IJ������� | |
| ��һ��ʵ�� | ��Ӧ�����а�������ɫ���塢gƿ�а����ͻ���ɫ���� |
| �ڶ���ʵ�� | ��Ӧ��������ɫ���壬gƿ�к���ɫ���̺ͻ���ɫ���� |
| ������ʵ�� | ��Ӧ��������ɫ���壬gƿ�л���ɫ���� |
�ٵ�һ��ʵ�飬����eƿû��ʢװ�κ�ҩƷ�����Եõ���ɫ���壬��ԭ���� ��
�ڵڶ���ʵ�飬gƿ�к���ɫ���̣���ԭ���� ��
��5��������ʵ��õ��Ĺ��壬����ܺ����������� �������Ҫ����Լ2��3mol/L��Ⱦ�ϻ�ԭ����Һ���������ȥ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
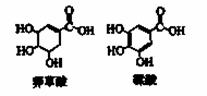
�����ᡢ�Ҵ�������������ˮ���ɵ�ƽ����ϵ�У�����������18O��ˮ��H218O��һ��ʱ����Լ�18O����Щ������
A.���ᡢ����������ˮ B.�Ҵ�������������ˮ
C.�Ҵ���ˮ D.���ᡢˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ����д�У���ȷ���� ��������
A���������ᷴӦ�� 2Fe��6H��===2Fe3����3H2��
B. NH4HCO3��Һ�����NaOH��Һ��Ӧ��NH4����OH��= NH3����H2O
C��AlCl3��Һ�м��������İ�ˮ�� Al3++ 3OH- �T�T Al(OH)3��
D. ̼��������Һ�е���ϡ��� HCO3- ��H+ = CO2 ��+H2O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com