
COŗĶNO¶¼ŹĒĘū³µĪ²ĘųÖŠµÄÓŠŗ¦ĪļÖŹ£¬ĖüĆĒÖ®¼äÄÜ»ŗĀżµŲ·¢ÉśČēĻĀ·“Ó¦£ŗ2NO(g)£«2CO(g)N2(g)£«2CO2(g)””¦¤H<0£¬ĻÖĄūÓĆ“Ė·“Ó¦£¬ÄāÉč¼ĘŅ»ÖÖ»·±£×°ÖĆ£¬ÓĆĄ“Ļū³żĘū³µĪ²Ęų¶Ō“óĘųµÄĪŪČ¾£¬ĻĀĮŠÉč¼Ę·½°øæÉŅŌĢįøßĪ²Ęų“¦ĄķŠ§¹ūµÄŹĒ (””””)”£
¢ŁŃ”ÓĆŹŹµ±µÄ“߻ƼĮ””¢ŚĢįøß×°ÖĆĪĀ¶Č””¢Ū½µµĶ×°ÖƵÄŃ¹Ēæ””¢Ü×°ÖĆÖŠ·ÅČė¼īŹÆ»Ņ
A£®¢Ł¢Ū”” B£®¢Ś¢Ü””
C£®¢Ł¢Ü”” D£®¢Ś¢Ū
½āĪö””¢ŁŃ”ÓĆŹŹµ±“߻ƼĮĖä²»ÄÜĢįøß·“Ó¦ĪļµÄ×Ŗ»ÆĀŹ£¬µ«ÄܼÓæģ·“Ó¦ĖŁĀŹ£¬ÕżČ·£»¢ŚŅņĪŖøĆ·“Ó¦µÄÕż·“Ó¦ĪŖ·ÅČČ·“Ó¦£¬ĖłŅŌÉżøßĪĀ¶Č£¬Ę½ŗāĻņÄę·“Ó¦·½ĻņŅĘ¶Æ£¬“ķĪ󣻢ŪøĆ·“Ó¦µÄÕż·“Ó¦ĪŖĘųĢåĢå»ż¼õŠ”µÄ·“Ó¦£¬Ņņ“Ė½µµĶŃ¹Ē棬ÄÜŹ¹Ę½ŗāĻņÄę·“Ó¦·½ĻņŅĘ¶Æ£¬“ķĪ󣻢Ü×°ÖĆÖŠ·ÅČė¼īŹÆ»ŅÄÜĪüŹÕCO2£¬Ź¹CO2µÄÅØ¶Č½µµĶ£¬Ę½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬ÕżČ·”£
“š°ø””C



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷ĻīĖłŹöµÄŹż×Ö²»ŹĒ6µÄŹĒ (””””)”£
A£®ŌŚNaCl¾§ĢåÖŠ£¬ÓėŅ»øöNa£«×ī½üµÄĒŅ¾ąĄėĻąµČµÄCl£µÄøöŹż
B£®ŌŚ½šøÕŹÆ¾§ĢåÖŠ£¬×īŠ”µÄ»·ÉĻµÄĢ¼Ō×ÓøöŹż
C£®ŌŚ¶žŃõ»Æ¹č¾§ĢåÖŠ£¬×īŠ”µÄ»·ÉĻµÄŌ×ÓøöŹż
D£®ŌŚŹÆÄ«¾§ĢåµÄʬ²ć½į¹¹ÖŠ£¬×īŠ”µÄ»·ÉĻµÄĢ¼Ō×ÓøöŹż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
A£®32 g O2Õ¼ÓŠµÄĢå»żŌ¼ĪŖ22.4 L
B£®22.4 L N2ŗ¬ÓŠ°¢·ü¼ÓµĀĀŽ³£ŹżøöµŖĘų·Ö×Ó
C£®ŌŚ±ź×¼×“æöĻĀ£¬22.4 LĖ®µÄÖŹĮæŌ¼ĪŖ18 g
D£®22 g¶žŃõ»ÆĢ¼Óė±ź×¼×“æöĻĀ11.2 L HClŗ¬ÓŠĻąĶ¬µÄ·Ö×ÓŹż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ź³ŃĪŹĒČĖĄąÉś»īÖŠ²»æÉȱɣµÄĪļÖŹ£¬ŗ£Ė®ÖŠŗ¬ÓŠ“óĮæŹ³ŃĪ”£
ijµŲ³ö²śµÄ“ÖŃĪÖŠ£¬Ėłŗ¬ŌÓÖŹŹĒCaCl2£¬ĶعżĻĀĆęµÄŹµŃéæÉÖĘµĆ“æ¾»µÄNaCl2”£
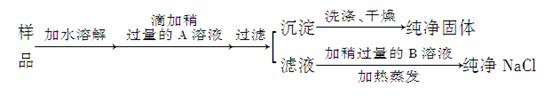 Ēė»Ų“š£ŗ
Ēė»Ų“š£ŗ
(1)¼ÓČėµÄAŹĒ__________£¬¼ģŃéAŅŃ¹żĮæµÄ·½·ØŹĒ_________________________
________________________________________________________________________ӣ
(2)¼ÓČėµÄBŹĒ__________£¬¼ÓČėÉŌ¹żĮæBµÄÄæµÄŹĒ________________________”£
(3)ĪŖ¼ģŃé³ĮµķŹĒ·ńĻ“¾»£¬×īŗĆŌŚ×īŗó¼øµĪĻ“³öŅŗÖŠ¼ÓČė______________________ČÜŅŗ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚĻĀĮŠø÷Ėµ·ØÖŠ£¬ÕżČ·µÄŹĒ(””””)
A£®¦¤H>0±ķŹ¾·ÅČČ·“Ó¦£¬¦¤H<0±ķŹ¾ĪüČČ·“Ó¦
B£®ČČ»Æѧ·½³ĢŹ½ÖŠµÄ»Æѧ¼ĘĮæŹżÖ»±ķŹ¾ĪļÖŹµÄĮ棬æÉŅŌŹĒ·ÖŹż
C£®1 mol H2SO4Óė1 mol Ba(OH)2·“Ӧɜ³ÉBaSO4³ĮµķŹ±·Å³öµÄČČ½Š×öÖŠŗĶČČ
D£®1 mol H2Óė0.5 mol O2·“Ó¦·Å³öµÄČČ¾ĶŹĒH2µÄČ¼ÉÕČČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĄūÓƵĶĪĀ¼¼ŹõæÉ“¦Ąķ·ĻĘųÖŠµÄµŖŃõ»ÆĪļ”£ŌŚŗćČŻĆܱÕČŻĘ÷ÖŠ·¢ÉśĻĀĮŠ»Æѧ·“Ó¦£ŗ
4NH3(g)£«6NO(g)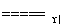 5N2(g)£«6H2O(l)
5N2(g)£«6H2O(l)
¦¤H£½Q kJ/mol(Q<0)”£ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ (””””)”£
A£®ĘäĖūĢõ¼ž²»±ä£¬Ź¹ÓĆøߊ§“߻ƼĮ£¬·ĻĘųÖŠµŖŃõ»ÆĪļµÄ×Ŗ»ÆĀŹŌö“ó
B£®Ę½ŗāŹ±£¬ĘäĖūĢõ¼ž²»±ä£¬Ōö¼ÓNH3µÄÅØ¶Č£¬·ĻĘųÖŠNOµÄ×Ŗ»ÆĀŹ¼õŠ”
C£®µ„Ī»Ź±¼äÄŚÉś³ÉNH3ŗĶH2OµÄĪļÖŹµÄĮæÖ®±ČĪŖ2”Ć3Ź±£¬·“Ó¦“ļµ½Ę½ŗā
D£®Ę½ŗāŹ±£¬ĘäĖūĢõ¼ž²»±ä£¬ÉżøßĪĀ¶ČæÉŹ¹øĆ·“Ó¦µÄĘ½ŗā³£ŹżŌö“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŗĻ³ÉĘųµÄÖ÷ŅŖ³É·ÖŹĒŅ»Ńõ»ÆĢ¼ŗĶĒāĘų£¬æÉÓĆÓŚŗĻ³É¼×ĆѵČĒå½ąČ¼ĮĻ”£ÓÉĢģČ»Ęų»ńµĆøĆŗĻ³ÉĘų¹ż³ĢÖŠæÉÄÜ·¢ÉśµÄ·“Ó¦ÓŠ£ŗ
¢ŁCH4(g)£«H2O(g)CO(g)£«3H2(g) ¦¤H1£½£«206.1 kJ”¤mol£1
¢ŚCH4(g)£«CO2(g)2CO(g)£«2H2(g) ¦¤H2£½£«247.3 kJ”¤mol£1
¢ŪCO(g)£«H2O(g)CO2(g)£«H2(g)””¦¤H3
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŌŚŅ»ĆܱÕČŻĘ÷ÖŠ½ųŠŠ·“Ó¦¢Ł£¬²āµĆCH4µÄĪļÖŹµÄĮæÅضČĖę·“Ó¦Ź±¼äµÄ±ä»ÆČēĶ¼1ĖłŹ¾”£

Ķ¼1
·“Ó¦½ųŠŠµÄĒ°5 minÄŚ£¬v(H2)£½________£»10 minŹ±£¬øıäµÄĶā½ēĢõ¼žæÉÄÜŹĒ______________________________________________________________”£
(2)ČēĶ¼2ĖłŹ¾£¬ŌŚ¼×”¢ŅŅĮ½ČŻĘ÷ÖŠ·Ö±š³äČėµČĪļÖŹµÄĮæµÄCH4ŗĶCO2£¬Ź¹¼×”¢ŅŅĮ½ČŻĘ÷³õŹ¼ČŻ»żĻąµČ”£ŌŚĻąĶ¬ĪĀ¶ČĻĀ·¢Éś·“Ó¦¢Ś£¬²¢Ī¬³Ö·“Ó¦¹ż³ĢÖŠĪĀ¶Č²»±ä”£ŅŃÖŖ¼×ČŻĘ÷ÖŠCH4µÄ×Ŗ»ÆĀŹĖꏱ¼äµÄ±ä»ÆČēĶ¼3ĖłŹ¾£¬ĒėŌŚĶ¼3ÖŠ»³öŅŅČŻĘ÷ÖŠCH4µÄ×Ŗ»ÆĀŹĖꏱ¼ä±ä»ÆµÄĶ¼Ļń”£

(3)·“Ó¦¢ŪÖŠ¦¤H3£½________”£800 ”ꏱ£¬·“Ó¦¢ŪµÄĘ½ŗā³£ŹżK£½1£¬²āµĆøĆĪĀ¶ČĻĀĆܱÕČŻĘ÷֊ijŹ±æĢø÷ĪļÖŹµÄĪļÖŹµÄĮæ¼ūĻĀ±ķ£ŗ
| CO | H2O | CO2 | H2 |
| 0.5 mol | 8.5 mol | 2.0 mol | 2.0 mol |
“ĖŹ±·“Ó¦¢ŪÖŠÕż”¢Äę·“Ó¦ĖŁĀŹµÄ¹ŲĻµŹ½ŹĒ________(Ģī“śŗÅ)”£
a£®vÕż>vÄę”” B£®vÕż<vÄę
c£®vÕż£½vÄę”” D£®ĪŽ·ØÅŠ¶Ļ
(4)ÓĆøĆŗĻ³ÉĘųÖĘČ”¼×ĆѵĻÆѧ·½³ĢŹ½ĪŖ______________________________”£
øĆ·“Ó¦µÄŌ×ÓĄūÓĆĀŹĪŖ________(Éč·“Ó¦ĶźČ«½ųŠŠ£¬ÓĆÖŹĮæ°Ł·Ö±Č±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ¹ŲÓŚpH±ä»ÆµÄÅŠ¶ĻÕżČ·µÄŹĒ(””””)
A£®ĪĀ¶ČÉż£¬Na2CO3ČÜŅŗpH¼õŠ”
B£®ĪĀ¶ČÉżøߣ¬“æĖ®pHŌö“ó
C£®ŠĀÖĘĀČĖ®¾¹āÕÕŅ»¶ĪŹ±¼äŗó£¬ČÜŅŗpH¼õŠ”
D£®ĒāŃõ»ÆÄĘČÜŅŗ¾ĆÖĆÓŚæÕĘųÖŠ£¬ČÜŅŗpH±ä“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½šŹōīŃŠŌÄÜÓÅŌ½£¬±»ÓžĪŖ¼ĢFe”¢AlŗóÓ¦ÓĆ¹ć·ŗµÄ”°µŚČż½šŹō”±”£
(1)Ti»łĢ¬Ō×ӵĵē×ÓÅŲ¼Ź½ĪŖ
(2)īŃÄÜÓėB”¢C”¢ N”¢ OµČ·Ē½šŹōŌŖĖŲŠĪ³ÉĪČ¶ØµÄ»ÆŗĻĪļ”£µēøŗŠŌ:C £ØĢī”°>”±»ņ”°<”±ĻĀĶ¬)B£»µŚŅ»µēĄėÄÜ£ŗ N O”£
(3)ŌĀĒņŃŅŹÆ——ŠžĪäŃŅµÄÖ÷ŅŖ³É·ÖĪŖīŃĖįŃĒĢś(FeTiO3) ”£ FeTiO3Óė80%µÄĮņĖį·“Ó¦æÉ Éś³ÉTiOSO4”£SO42-µÄæռ乹ŠĶĪŖ ŠĪ£¬ĘäÖŠĮņŌ×Ó²ÉÓĆ ŌÓ»Æ.
(4)Į×ĖįīŃ”²Ti3 (PO4 )4]ļ®Ąė×Óµē³ŲÄÜĮæĆÜ¶Č“ó”¢°²Č«ŠŌøß”£Ti3 (PO4 )4æÉÓÉTiOSO4Óė
 H3PO4·“Ó¦ÖʵƔ£Į½·Ö×ÓH3PO4·¢ÉśĶŃĖ®Éś³É½¹Į×Ėį£»
H3PO4·“Ó¦ÖʵƔ£Į½·Ö×ÓH3PO4·¢ÉśĶŃĖ®Éś³É½¹Į×Ėį£»
ŌņČż·Ö×ÓH3PO4ĶŃČ„Į½·Ö×ÓH2OÉś³ÉČżĮ×Ėį£¬Ęä½į¹¹Ź½ĪŖ £¬ĖÄ·Ö×ÓH3PO4 ĶŃČ„ĖÄ·Ö×ÓH2OÉś³ÉµÄĖÄĘ«Į×ĖįŹōÓŚ ŌŖĖį”£
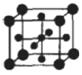
(5)TiµÄŃõ»ÆĪļŗĶCaOĻą»„×÷ÓĆÄÜŠĪ³ÉīŃĖįŃĪCaTiO3£¬CaTiO3µÄ¾§Ģå½į¹¹ČēĶ¼ĖłŹ¾(Ti4+Ī»ÓŚĮ¢·½ĢåµÄ¶„µć)”£øĆ¾§ĢåÖŠ£¬Ti4+ŗĶÖÜĪ§ øöO2-Ļą½ōĮŚ.
(6)FeÄÜŠĪ³É¶ąÖÖŃõ»ÆĪļ£¬ĘäÖŠFeO¾§°ū½į¹¹ĪŖNaCIŠĶ”£¾§ĢåÖŠŹµ¼ŹÉĻ“ęŌŚæÕĪ»”¢“ķĪ»”¢ŌÓÖŹŌ×ÓµČȱĻŻ£¬¾§ĢåȱĻŻ¶Ō¾§ĢåµÄŠŌÖŹ»į²śÉśÖŲ“óÓ°Ļģ”£ÓÉÓŚ¾§ĢåȱĻŻ£¬ŌŚ¾§ĢåÖŠFeŗĶOµÄøöŹż±Č·¢ÉśĮĖ±ä»Æ£¬±äĪŖFexO£Øx<1£©ÖŠ£¬Čō²āµĆijFexO¾§ĢåĆܶČĪŖ5.71 g·cm-3£¬¾§°ū±ß³¤ĪŖ4.28X10-10 m,ŌņFexOÖŠx=_”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com