
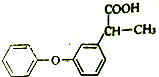
 Ēė°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ
Ēė°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ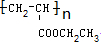 £®
£® ·ÖĪö £Ø1£© ŗ¬-COOH”¢-C-O-C£¬æÉ·¢ÉśČ”“ś”¢¼Ó³É·“Ó¦£»
ŗ¬-COOH”¢-C-O-C£¬æÉ·¢ÉśČ”“ś”¢¼Ó³É·“Ó¦£»
£Ø2£©¢ŁŅŅĻ©ŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬æÉÓėĖ®·¢Éś¼Ó³É·“Ӧɜ³ÉŅŅ“¼£»
¢ŚCH2=CHCOOCH2CH3ŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬æÉ·¢Éś¼Ó¾Ū·“Ó¦£®
½ā“š ½ā£ŗ£Ø1£©ÓŠ»śĪļŗ¬ÓŠµÄ¹ŁÄÜĶÅĪŖōČ»łŗĶĆŃ»ł£¬ĒŅŗ¬ÓŠ±½»·£¬æÉ·¢Éś¼Ó³É”¢Č”“ś£Øõ„»Æ·“Ó¦£©£¬¹Ź“š°øĪŖ£ŗōČ»łŗĶĆŃ»ł£»¼Ó³É”¢Č”“ś·“Ó¦£»
£Ø2£©¢ŁCH2=CH2ÖŠ²»±„ŗĶµÄC=CĖ«¼üÖŠĘäÖŠ1øöC-C¶ĻĮŃ½įŗĻĖ®Ģį¹©µÄ-H”¢-OH£¬Éś³ÉŅŅ“¼£¬·½³ĢŹ½ĪŖ£ŗCH2=CH2+H2O$”ś_{”÷}^{“߻ƼĮ}$ CH3CH2OH£¬
¹Ź“š°øĪŖ£ŗCH2=CH2+H2O$”ś_{”÷}^{“߻ƼĮ}$ CH3CH2OH£»
¢ŚCH2=CHCOOCH2CH3µÄ¾ŪŗĻ·“Ó¦ĪŖnCH2=CHCOOCH2CH3$\stackrel{“߻ƼĮ}{”ś}$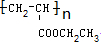 £¬¹Ź“š°øĪŖ£ŗnCH2=CHCOOCH2CH3$\stackrel{“߻ƼĮ}{”ś}$
£¬¹Ź“š°øĪŖ£ŗnCH2=CHCOOCH2CH3$\stackrel{“߻ƼĮ}{”ś}$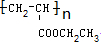 £®
£®
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļµÄ½į¹¹ŗĶŠŌÖŹ£¬ĪŖøßĘµæ¼µć£¬²ąÖŲӌѧɜµÄ·ÖĪöÄÜĮ¦µÄ漲飬עŅā°ŃĪÕÓŠ»śĪļµÄ½į¹¹ŗĶ¹ŁÄÜĶŵĊŌÖŹ£¬°ŃĪÕÓŠ»śĪļµÄĆüĆū·½·Ø£¬ÄŃ¶Č²»“ó£®


 æŚĖćĢāæؼÓÓ¦ÓĆĢā¼ÆѵĻµĮŠ“š°ø
æŚĖćĢāæؼÓÓ¦ÓĆĢā¼ÆѵĻµĮŠ“š°ø ×ŪŗĻ×Ō²āĻµĮŠ“š°ø
×ŪŗĻ×Ō²āĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
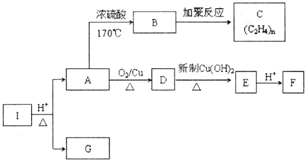 »ÆŗĻĪļIµÄ·Ö×ÓŹ½ĪŖC6H10O4£¬75%µÄAČÜŅŗ³£ÓĆÓŚŅ½ĮĘĻū¶¾£¬ÓėIĻą¹ŲµÄ·“Ó¦ČēĶ¼£®øł¾ŻŅŌÉĻŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£®
»ÆŗĻĪļIµÄ·Ö×ÓŹ½ĪŖC6H10O4£¬75%µÄAČÜŅŗ³£ÓĆÓŚŅ½ĮĘĻū¶¾£¬ÓėIĻą¹ŲµÄ·“Ó¦ČēĶ¼£®øł¾ŻŅŌÉĻŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£®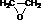 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ²»É÷½«ÅؼīČÜŅŗÕ“µ½Ę¤·ōÉĻ£¬Į¢¼“ÓĆ“óĮæĮņĖį³åĻ“£¬Č»ŗóŌŁÓĆĖ®³åĻ“ | |
| B£® | ²»É÷½«Ėį½¦µ½ŃŪÖŠ£¬Į¢¼“ÓĆ“óĮæĖ®³åĻ“£¬±ßĻ“±ßÕ£ŃŪ¾¦ | |
| C£® | ŹµŃ鏱ŹÖÖøÉĻ²»Š”ŠÄÕ“ÉĻÅØĮņĖį£¬Į¢¼“ÓĆNaOHČÜŅŗĒåĻ“ | |
| D£® | ¾Ę¾«²»É÷Č÷ŌŚŹµŃéץÉĻÉÕĘšĄ“Ź±£¬Į¢¼“ÓĆĖ®½½Ćš |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĀČĘųČÜÓŚĖ®£ŗCl2+H2OØT2H++Cl-+ClO- | |
| B£® | ĻņCuSO4ČÜŅŗÖŠ¼ÓČėNaOH£ŗCu2++2OH-ØTCu£ØOH£©2”ż | |
| C£® | ŌŚĖįŠŌČÜŅŗÖŠ£¬KIO3ÓėKI·“Ӧɜ³ÉI2£ŗIO3-+I-+6H+ØTI2+3H2O | |
| D£® | ĻņAl2£ØSO4£©3ČÜŅŗÖŠ¼ÓČė¹żĮæµÄNH3?H2O2£ŗAl3++4NH3?H2O2ØTAlO2-+4NH4++2H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
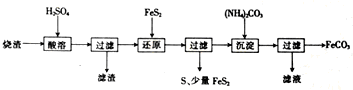
| Ąė×Ó | Ąė×ÓÅØ¶Č£Ømol?L-1£© | |
| »¹ŌĒ° | »¹Ōŗó | |
| Fe2+ | 0.10 | 2.50 |
| SO42- | 3.50 | 3.70 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2.8g | B£® | 5.6g | C£® | 8.8g | D£® | 11.2g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
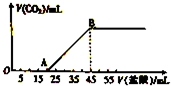 ½«5.08gÓÉNa2CO3ŗĶNaHCO3×é³ÉµÄ¹ĢĢå»ģŗĻĪļĶźČ«ČÜÓŚĖ®£¬ÖĘ³ÉČÜŅŗ£¬Č»ŗóĻņøĆČÜŅŗÖŠÖšµĪ¼ÓČė2mol?L-1µÄŃĪĖį£¬Ėł¼ÓČėŃĪĖįµÄĢå»żÓė²śÉśCO2Ģå»ż£Ø±ź×¼×“æö£©µÄ¹ŲĻµČēĶ¼ĖłŹ¾£®
½«5.08gÓÉNa2CO3ŗĶNaHCO3×é³ÉµÄ¹ĢĢå»ģŗĻĪļĶźČ«ČÜÓŚĖ®£¬ÖĘ³ÉČÜŅŗ£¬Č»ŗóĻņøĆČÜŅŗÖŠÖšµĪ¼ÓČė2mol?L-1µÄŃĪĖį£¬Ėł¼ÓČėŃĪĖįµÄĢå»żÓė²śÉśCO2Ģå»ż£Ø±ź×¼×“æö£©µÄ¹ŲĻµČēĶ¼ĖłŹ¾£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ijĶ¬Ń§×é×°ĮĖČēĶ¼ĖłŹ¾µÄµē»ÆѧװÖĆ£®µē¼«¢ńĪŖAl£¬ĘäĖūµē¼«¾łĪŖCu£®
ijĶ¬Ń§×é×°ĮĖČēĶ¼ĖłŹ¾µÄµē»ÆѧװÖĆ£®µē¼«¢ńĪŖAl£¬ĘäĖūµē¼«¾łĪŖCu£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 µÄĮ“½ŚĪŖ
µÄĮ“½ŚĪŖ £®Ę䵄ĢåµÄ½į¹¹¼ņŹ½·Ö±šĪŖ
£®Ę䵄ĢåµÄ½į¹¹¼ņŹ½·Ö±šĪŖ ”¢
”¢ £®
£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com