
“Ó·Ļ·°“߻ƼĮ£ØÖ÷ŅŖ³É·ÖV2O5”¢VOSO4”¢K2SO4”¢SiO2µČ£©ÖŠ»ŲŹÕV2O5µÄŅ»ÖÖÉś²ś¹¤ŅÕĮ÷³ĢŹ¾ŅāĶ¼ČēĻĀ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©²½Öč¢ŁÖŠ·ĻŌüµÄÖ÷ŅŖ³É·ÖŹĒ £¬¢ŪÖŠXŹŌ¼ĮĪŖ ”£
£Ø2£©ŹµŃéŹŅÖŠ½ųŠŠŻĶČ”·ÖŅŗ²Ł×÷Ź±£¬×¢ČėŻĶČ”¼Į£¬³ä·ÖÕńµ“£¬½«·ÖŅŗĀ©¶·ÓŚĢśČ¦ÉĻ¾²ÖĆ£¬µ±ŅŗĢå·Ö²ćŗ󣬽ÓĻĀĄ“µÄ²Ł×÷ŹĒ ”£
£Ø3£©¢Ś”¢¢ŪµÄ±ä»Æ¹ż³Ģæɼņ»ÆĪŖ£ØĻĀŹ½R±ķŹ¾VO2+£¬HA±ķŹ¾ÓŠ»śŻĶČ”¼Į£©”£
R2(SO4)n (Ė®²ć)+ 2nHA£ØÓŠ»ś²ć£© 2RAn£ØÓŠ»ś²ć£© + nH2SO4 (Ė®²ć)ĪŖĢįøߢŚÖŠŻĶČ”°Ł·ÖĀŹ£¬Ó¦²ÉČ”µÄ“ėŹ©ŹĒ ”£
2RAn£ØÓŠ»ś²ć£© + nH2SO4 (Ė®²ć)ĪŖĢįøߢŚÖŠŻĶČ”°Ł·ÖĀŹ£¬Ó¦²ÉČ”µÄ“ėŹ©ŹĒ ”£
£Ø4£©ĒėĶź³É¢ÜÖŠµÄ·“Ó¦Ąė×Ó·½³ĢŹ½£ŗ
”õClO3- + ”õVO2+ +”õH+ =”õVO3+ + ”õ +”õ
£Ø5£©25”ꏱ£¬Č”Ńł½ųŠŠŹŌŃé·ÖĪö£¬µĆµ½·°³ĮµķĀŹŗĶČÜŅŗpHÖ®¼ä¹ŲĻµČēĻĀ±ķ£ŗ
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ·°³ĮµķĀŹ% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
¢ÅSiO2£Ø2·Ö£© H2SO4 £Ø2·Ö£©
¢ĘŌŚ·ÖŅŗĀ©¶·ĻĀ·½·ÅŅ»½ą¾»ÉÕ±£ØŹ¹Ā©¶·ĻĀ¶Ė¹ÜæŚ½ōææÉÕ±ÄŚ±Ś£©£¬“ņæŖ·ÖŅŗĀ©¶·ÉĻæŚČū×ӣػņ½«·ÖŅŗĀ©¶·ÉĻæŚČū×Ó°¼²Ū¶Ō×¼Ā©¶·æŚ¾±ÉĻŠ”æ×£©£¬“ņæŖ»īČū£¬½«ĻĀ²ćŅŗĢå·ÅČėÉÕ±£¬µ±·ÖŅŗĆę½Ó½ü£Ø»ņĀŌ³¬¹ż£©»īČū“¦Ź±¹Ų±Õ»īČū£»ÉĻ²ćŅŗĢå“Ó·ÖŅŗĀ©¶·ÉĻæŚµ¹ČėĮķŅ»ÉÕ±ÖŠ”££Ø2·Ö£©ŅŖµć£ŗ“ņæŖ·ÖŅŗĀ©¶·ÉĻæŚČū×ӣػņ½«·ÖŅŗĀ©¶·ÉĻæŚČū×Ó°¼²Ū¶Ō×¼Ā©¶·æŚ¾±ÉĻŠ”æ×£©£Ø1·Ö£©£»ĻČ“ņæŖ»īČū·Å³öĻĀ²ćŅŗĢ壬ŗó½«ÉĻ²ćŅŗĢå“Ó·ÖŅŗĀ©¶·ÉĻæŚµ¹ČėĮķŅ»ÉÕ±ÖŠ£Ø1·Ö£©”£
¢Ē¼ÓČė¼īÖŠŗĶĮņĖįŹ¹Ę½ŗāÕżŅĘ”¢¶ą“ĪĮ¬ŠųŻĶČ”£Ø2·Ö£© Š“”°¼ÓČė¼ī£ØæÉŅŌŹĒNaOH”¢KOH”¢°±Ė®£©ÖŠŗĶĮņĖįŹ¹Ę½ŗāÕżŅĘ”±£¬µĆ2·Ö£»Š“”°¶ą“ĪĮ¬ŠųŻĶČ””±£¬µĆ2·Ö£»Š“”°¼ÓČėMg”¢Fe”¢ČõĖįĒæ¼īŃĪ”±£¬¶¼øų2·Ö£»Š“”°Ōö“óŻĶČ”¼ĮµÄ¼ÓČėĮæ”±£¬øų1·Ö£¬Š“”°Ōö“óŻĶČ”¼ĮÅØ¶Č”±²»µĆ·Ö£»Š“”°ŅĘ³öH2SO4 ”±øų1·Ö”£
¢Č1ClO3- + 6VO2+ + 6H+ = 6VO3+ + 1Cl- + 3H2O £Ø2·Ö£©²¹³öCl-ŗĶH2O£¬1·Ö”£ÅäĘ½ÕżČ·£¬1·Ö”£
²»Š“”°1”±£¬æŪ1·Ö”£
¢É1.7”Ŗ1.8£Ø2·Ö£© Š“1.7”¢1.8”¢1.75”¢1.7”Ŗ1.8Ö®¼ä¾łøų2·Ö”£
Š“³É1.6£¬øų1·Ö”£
¢Ź°±Ęų ÓŠ»śŻĶČ”¼Į£Ø2·Ö£©ø÷1·Ö”£Ćæ¶ąŠ“Ņ»øöæŪ1·Ö”£
½āĪöŹŌĢā·ÖĪö£ŗ¹¤ŅÕĮ÷³ĢĢāµÄ¹Ų¼üŹĒøćĒ峞¼ÓČėŹ²Ć“ĪļÖŹ£¬·¢ÉśŹ²Ć“·“Ó¦£¬ČēŗĪ·ÖĄėµČĪŹĢā£¬½«¹¤ŅÕĮ÷³Ģ×Ŗ»ÆĪŖĪļÖŹµÄĮ÷³Ģ”£¢ŁSiO2²»ČÜÓŚĖ®”¢Ėį£¬Ķعż¹żĀĖ³żČ„£¬ŌŚ·ĻŌüÖŠ£¬V2O5±»»¹ŌĪŖVO2+£¬ÓėĘäĖūĄė×ÓŅ»Ęš“ęŌŚŌŚĀĖŅŗÖŠ£»¢Ś“Ó·ÖĄėÖŠæÉŅŌ擳ö£¬VO2+ŅŌČÜÓŚÓŠ»śČܼĮ£¬ĶعżŻĶČ”·ÖĄė£»¢ŪĶعż·“ŻĶČ”£¬µĆµ½ĖįŠŌĖ®ČÜŅŗŗ¬VO2+”¢SO42-£¬¹ŹXĪŖH2SO4£»Č»ŗóĶعżŃõ»Æ½«V“Ó+4µ½+5£¬¼ÓČė°±Ė®£¬³Ź¼īŠŌ×Ŗ»ÆĪŖ³Įµķ£¬Ķعż±ŗÉÕ£¬µĆµ½V2O5”£Õūøö¹¤ŅÕĮ÷³Ģ²¢²»ø“ŌÓ”£
æ¼µć£ŗŅŌ¹¤ŅÕĮ÷³ĢĪŖ»ł“”£¬æ¼²é»ÆѧÓė¹¤ŅµµÄ¹ŲĻµ£¬×¢ÖŲŌŖĖŲ»ÆŗĻĪļÖŖŹ¶”¢ŹµŃé²Ł×÷”¢Ńõ»Æ»¹Ō·“Ó¦µÄ漲锣



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¶žŃõ»Æīę£ØCeO2£©ŹĒŅ»ÖÖÖŲŅŖµÄĻ”ĶĮŃõ»ÆĪļ”£Ę½°åµēŹÓĻŌŹ¾ĘĮÉś²ś¹ż³ĢÖŠ²śÉś“óĮæµÄ·Ļ²£Į§·ŪÄ©£Øŗ¬SiO2”¢Fe2O3”¢CeO2µČĪļÖŹ£©”£Ä³æĪĢāŅŌ“Ė·ŪÄ©ĪŖŌĮĻ£¬×ŹŌ“»ŲŹÕµÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ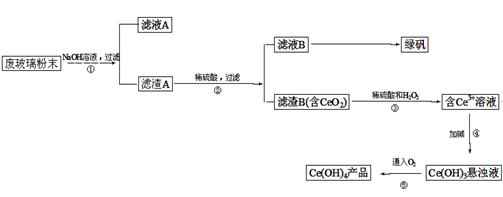
£Ø1£©Š“³öµŚ¢Ł²½·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
£Ø2£©Ļ“µÓĀĖŌüBµÄÄæµÄŹĒĪŖĮĖ³żČ„____£ØĢīĄė×Ó·ūŗÅ£©£¬¼ģŃéøĆĄė×ÓŹĒ·ńĻ“¾»µÄ·½·ØŹĒ ”£
£Ø3£©Š“³öµŚ¢Ū²½·“Ó¦µÄ»Æѧ·½³ĢŹ½_____________________________”£
£Ø4£©ÖʱøĀĢ·Æ(FeSO4”¤7H2O)Ź±£¬ĻņFe2(SO4)3ČÜŅŗÖŠ¼ÓČė¹żĮæĢśŠ¼£¬³ä·Ö·“Ó¦ŗ󣬾¹żĀĖµĆµ½FeSO4ČÜŅŗ£¬ŌŁ¾ ”¢ ”¢¹żĀĖ”¢Ļ“µÓ”¢øÉŌļµČ²Ł×÷²½ÖčµĆµ½ĀĢ·Æ”£
£Ø5£©Č”ÉĻŹöĮ÷³ĢÖŠµĆµ½µÄCe(OH)4²śĘ·(ÖŹĮæ·ÖŹżĪŖ97%)1.0g£¬¼ÓĮņĖįČܽāŗó£¬ÓĆ0.1000mol/LFeSO4ČÜŅŗµĪ¶ØÖĮÖÕµć£Øīę±»»¹Ō³ÉCe3+)£¬ŌņŠč×¼Č·µĪ¼Ó±ź×¼ČÜŅŗµÄĢå»żĪŖ mL”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ij°×É«·ŪÄ©ÓÉĢ¼ĖįÄĘ”¢ĻõĖįĆ¾”¢ĮņĖįĶ”¢ĀČ»Æ¼Ų”¢ĀČ»Æļ§ÖŠµÄŅ»ÖÖ»ņ¼øÖÖ×é³É”£ĪŖĮĖ¼ģŃéĖüĆĒĖłŗ¬µÄĪļÖŹ£¬×öĮĖŅŌĻĀŹµŃ锣¢ŁČ”²æ·Ö·ŪÄ©£¬¼ÓĖ®Čܽā£¬µĆĪŽÉ«ČÜŅŗ”£½«ĖłµĆČÜŅŗ·Ö³ÉĮ½·Ż£¬·Ö±š½ųŠŠŹµŃ飻¢ŚŌŚµŚŅ»·ŻČÜŅŗÖŠµĪ¼Ó×ćĮæĻ”ŃĪĖį£¬ÓŠĘųÅŻ²śÉś£¬¼ĢŠųĶł·“Ó¦ŗóµÄČÜŅŗÖŠµĪ¼ÓAgNO3ČÜŅŗÓŠ°×É«³ĮµķÉś³É£»¢ŪŌŚµŚ¶ž·ŻČÜŅŗÖŠµĪ¼ÓĒāŃõ»ÆÄĘČÜŅŗ²¢¼ÓČČ£¬½«ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½·ÅŌŚŹŌ¹ÜæŚ£¬ŹŌÖ½±äĄ¶”£ÓÉ“ĖæÉÅŠ¶Ļ¹ĢĢå»ģŗĻĪļÖŠæĻ¶Øŗ¬ £ØŠ“»ÆѧŹ½£¬ĻĀĶ¬£©£¬æĻ¶Øƻӊ £¬æÉÄÜŗ¬ÓŠ ”£¶ŌæÉÄÜÓŠµÄĪļÖŹ£¬æɲÉÓĆ Ą“¼ģŃ飬Čē¹ūŗ¬ÓŠøĆĪļÖŹ£¬ĘäĻÖĻóŹĒ ”£²½Öč¢ŪÖŠÓŠ¹ŲµÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
“Ó»ŲŹÕµÄŗ¬ĶµēĄĀ·ĻĮĻÖŠĢįČ”ĶŹ±£¬¼ŁČōÉč¼ĘČēĻĀĮ½ÖÖ·½°ø£¬»Ų“šÓŠ¹ŲĪŹĢā”£
·½°ø¼×£ŗ
·½°øŅŅ£ŗ
£Ø1£©Į½øö·½°øÖŠ£¬·ūŗĻµ±Ē°Éś²śÖŠĀĢÉ«»ÆѧĄķÄīµÄŹĒ·½°ø £¬ĄķÓÉŹĒ ·½°øµŚŅ»²½”°×ĘÉÕ”±»į²śÉśĪŪČ¾æÕĘųµÄĘųĢ唢·Ū³¾”¢ŃĢĪķ”£
£Ø2£©·½°øŅŅÖŠĶČܽāÉś³ÉĄ¶É«ČÜŅŗŹ±·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________________ _ ______”£
£Ø3£©ĪŖĮĖĢįøßŌĮĻµÄĄūÓĆĀŹ,×īŗóŅ»²½ĖłµĆĒ³ĀĢÉ«ĀĖŅŗĶعżÕō·¢ÅØĖõ”¢ĄäČ“½į¾§”¢¹żĀĖ”¢Ļ“µÓ”¢×ŌČ»øÉŌļæɵƵ½Ņ»ÖÖ½į¾§Ė®ŗĻĪļµÄ¾§Ģ唣»ńµĆ¾§Ģåŗó¶ŌĘä½ųŠŠ¼ģ²ā£ŗ
¢ŁĻČČ”a gµÄ¾§Ģå½ųŠŠĶŃĖ®ŹµŃ飬»ńµĆĪŽĖ®¹ĢĢåĪŖ£Øa”Ŗ1.26£©g
¢Ś½«ĪŽĖ®¹ĢĢåČÜÓŚ×ćĮæµÄĖ®Åä³ÉČÜŅŗŗóµĪ¼Ó1.00mol/LµÄĀČ»Æ±µČÜŅŗ£¬µ±µĪ¼Ó10.00mLČÜŅŗŹ±£¬³ĮµķĒ”ŗĆĶźČ«”£Ķعż¼ĘĖć²āÖŖøĆ¾§ĢåµÄ»ÆѧŹ½ŹĒ ”£
£Ø4£©ĀČ»ÆŃĒĶ£ØCuCl£©ŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£¹ś¼Ņ±ź×¼¹ę¶ØŗĻøńµÄCuCl²śĘ·µÄÖ÷ŅŖÖŹĮæÖø±źĪŖCuClµÄÖŹĮæ·ÖŹż“óÓŚ96.5% ”£¹¤ŅµÉĻÓĆĮņĖįĶµČŌĮĻ³£ĶعżĻĀĮŠ·“Ó¦ÖʱøCuCl £ŗ
2CuSO4+ Na2SO3 + 2 NaCl + Na2CO3£½2 CuCl ”ż+ 3 Na2SO4 + CO2”ü
²ā¶ØCuClÖŹĮæ·ÖŹżŹ±ĻČ×¼Č·³ĘČ”ĖłÖʱøµÄ0.2500g CuClѳʷÖĆÓŚŅ»¶ØĮæµÄ0.5mol”¤L-1 FeCl3ČÜŅŗÖŠ£¬“żŃłĘ·ĶźČ«Čܽāŗ󣬼ÓĖ®20mL£¬ÓĆ0.1000mol”¤L-1 Ce£ØSO4£©2ČÜŅŗµĪ¶Øµ½ÖÕµć£¬ĻūŗÄ24.60mL Ce£ØSO4£©2ČÜŅŗ”£ÓŠ¹Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗFe 3++CuCl£½Fe 2++Cu2++Cl£ £¬Ce4+ + Fe 2+£½Fe 3+ + Ce3+
¼ĘĖćÉĻŹöѳʷ֊CuClµÄÖŹĮæ·ÖŹżĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĪŖĮĖ“ļµ½ĻĀĮŠ±ķøńÖŠµÄŹµŃéŅŖĒó£¬Ēė“Ó¹©Ń”ŌńµÄ»ÆѧŹŌ¼Į¼°ŹµŃé·½·ØÖŠŃ”³öŗĻŹŹµÄ£¬½«Ę䱟ŗÅĢīČė¶ŌÓ¦µÄæÕøńÖŠ”£
| ŹµŃéŅŖĒó | ŹŌ¼Į¼°·½·Ø |
| Ö¤Ć÷Ć÷·ÆĖ®ČÜŅŗ³ŹĖįŠŌ | |
| ¼ų±š¼×ĶéÓėŅŅĻ© | |
| ³żČ„MgOÖŠŗ¬ÓŠµÄAl2O3 | |
| ¼ų±šŅŅ“¼ŗĶŅŅČ© | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
³£¼ūµÄĪåÖÖŃĪA”¢B”¢C”¢D”¢E£¬ĖüĆĒµÄŃōĄė×ÓæÉÄÜŹĒNa£«”¢NH4+”¢Cu2£«”¢Ba2£«”¢Al3£«”¢Ag£«”¢Fe3£«£¬ŅõĄė×ÓæÉÄÜŹĒCl£”¢NO3-”¢SO42-”¢CO32-£¬ŅŃÖŖ£ŗ
¢ŁĪåÖÖŃĪ¾łČÜÓŚĖ®£¬Ė®ČÜŅŗ¾łĪŖĪŽÉ«”£
¢ŚDµÄŃęÉ«·“Ó¦³Ź»ĘÉ«”£
¢ŪAµÄČÜŅŗ³ŹÖŠŠŌ£¬B”¢C”¢EµÄČÜŅŗ³ŹĖįŠŌ£¬DµÄČÜŅŗ³Ź¼īŠŌ”£
¢ÜČōŌŚÕāĪåÖÖŃĪµÄČÜŅŗÖŠ·Ö±š¼ÓČėBa(NO3)2ČÜŅŗ£¬Ö»ÓŠA”¢CµÄČÜŅŗ²»²śÉś³Įµķ”£
¢ŻČōŌŚÕāĪåÖÖŃĪµÄČÜŅŗÖŠ£¬·Ö±š¼ÓČė°±Ė®£¬EŗĶCµÄČÜŅŗÖŠÉś³É³Įµķ£¬¼ĢŠų¼Ó°±Ė®£¬CÖŠ³ĮµķĻūŹ§”£
¢Ž°ŃAµÄČÜŅŗ·Ö±š¼ÓČėµ½B”¢C”¢EµÄČÜŅŗÖŠ£¬¾łÄÜÉś³É²»ČÜÓŚĻ”ĻõĖįµÄ³Įµķ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĪåÖÖŃĪÖŠ£¬Ņ»¶ØƻӊµÄŃōĄė×ÓŹĒ £»Ėłŗ¬ŅõĄė×ÓĻąĶ¬µÄĮ½ÖÖŃĪµÄ»ÆѧŹ½ŹĒ ”£
£Ø2£©DµÄ»ÆѧŹ½ĪŖ £¬DČÜŅŗĻŌ¼īŠŌµÄŌŅņŹĒ(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾) ”£
£Ø3£©EŗĶ°±Ė®·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”£
£Ø4£©Éč¼ĘŹµŃé¼ģŃéBÖŠĖłŗ¬µÄŃōĄė×Ó£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ė®ŹĒŅ»ÖÖÖŲŅŖµÄ×ŌȻ׏Ō“£¬ŹĒČĖĄąĄµŅŌÉś“ę²»æÉȱɣµÄĪļÖŹ”£Ė®ÖŹÓÅĮÓÖ±½ÓÓ°ĻģČĖĢ彔浔£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĢģČ»Ė®ÖŠČܽāµÄĘųĢåÖ÷ŅŖÓŠ ”¢ £ØĢī»ÆѧŹ½£©”£
£Ø2£©¼ģŃéÕōĮóĖ®µÄ“æ¶ČŹ±£¬×ī¼ņµ„Ņ׊ŠµÄ·½·ØŹĒ²ā¶ØĖ®µÄ ”£
£Ø3£©Ė®µÄ¾»»ÆÓėČķ»ÆµÄĒų±šŹĒ£ŗĖ®µÄ¾»»ÆŹĒÓĆ»ģÄż¼Į£ØČēĆ÷·ÆµČ£©Ź¹ _£¬¶ųĖ®µÄČķ»ÆŹĒ________”£
£Ø4£©ĶعżŹ©¼ÓŅ»¶ØŃ¹Į¦Ź¹Ė®·Ö×ÓĶø¹ż°ėĶøĤ¶ų½«“ó·Ö×Ó»ņĄė×Ó½ŲĮō£¬“Ó¶ųŹ¹Ė®µĆŅŌ¾»»ÆµÄ·½·Ø³ĘĪŖ £»µēÉųĪö·Ø¾»»ÆĖ®Ź±£¬Ź¹Ąė×ÓĶعż°ėĶøĤµÄĶʶÆĮ¦ŹĒ ”£
£Ø5£©Ä³ĢģČ»Ė®ÖŠ ”¢
Ӣ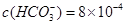
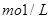 ”£ŅŖČķ»Æ10 m3ÕāÖÖĢģČ»Ė®£¬ŠčĻČ¼ÓČėCa£ØOH£©2 g£¬ŌŁ¼ÓČėNa2CO3 g”£
”£ŅŖČķ»Æ10 m3ÕāÖÖĢģČ»Ė®£¬ŠčĻČ¼ÓČėCa£ØOH£©2 g£¬ŌŁ¼ÓČėNa2CO3 g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
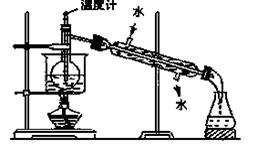
£Ø9·Ö£©ŗ£ŃóÖ²Īļ£¬Čēŗ£“ų”¢ŗ£ŌåÖŠŗ¬ÓŠ·įø»µÄµāŌŖĖŲ£¬µāŌŖĖŲŅŌµāĄė×ӵĊĪŹ½“ęŌŚ”£ŹµŃéŹŅĄļ“Óŗ£“ųÖŠĢįČ”µāµÄĮ÷³ĢČēĻĀ£ŗ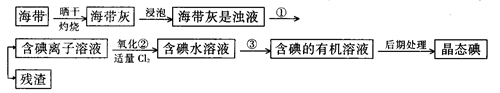
£Ø1£©Öø³öĢįČ”µāµÄ¹ż³ĢÖŠÓŠ¹ŲµÄŹµŃé²Ł×÷Ćū³Ę£ŗ
¢Ł £»¢Ū______________________”£
Š“³öŹµŃé¢ŚÖŠÓŠ¹Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½ _______________________ ”£
£Ø2£©ĢįČ”µāµÄ¹ż³ĢÖŠ£¬æɹ©Ń”ŌńµÄÓŠ¹ŲŹŌ¼ĮŹĒ___________”£
| A£®¼×±½£¬¾Ę¾« | B£®ĖÄĀČ»ÆĢ¼£¬±½ |
| C£®ĘūÓĶ£¬ŅŅĖį | D£®ĘūÓĶ£¬øŹÓĶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
4,7-¶ž¼×»łĻć¶¹ĖŲ£ØČŪµć£ŗ132.6”ę£©ŹĒŅ»ÖÖÖŲŅŖµÄĻćĮĻ£¬¹ć·ŗ·Ö²¼ÓŚÖ²Īļ½ēÖŠ,Óɼä¼×±½·ÓĪŖŌĮĻµÄŗĻ³É·“Ó¦ČēĻĀ£ŗ
ŹµŃé×°ÖĆĶ¼ČēĻĀ£ŗ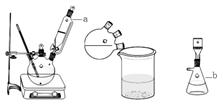
Ö÷ŅŖŹµŃé²½Öč£ŗ
²½Öč1.ĻņČżæŚÉÕĘæÖŠ¼ÓČė60mLÅØĮņĖį£¬²¢ĄäČ“ÖĮ0”ęŅŌĻĀ£¬½Į°čĻĀµĪČė¼ä¼×±½·Ó30mL(0.29mol)ŗĶŅŅõ£ŅŅĖįŅŅõ„26.4mL (0.21mol)µÄ»ģŗĻĪļ”£
²½Öč2.±£³ÖŌŚ10”ęĻĀ£¬½Į°č12h£¬·“Ó¦ĶźČ«ŗ󣬽«Ęäµ¹Čė±łĖ®»ģŗĻĪļÖŠ£¬Č»ŗó³éĀĖ”¢Ė®Ļ“µĆ“ÖĘ·
²½Öč3.“ÖĘ·ÓĆŅŅ“¼Čܽā²¢ÖŲ½į¾§£¬µĆ°×É«Õėד¾§Ģå²¢ŗęøÉ£¬³ĘµĆ²śĘ·ÖŹĮæĪŖ33.0g”£
£Ø1£©Ķ¼ÖŠŅĒĘ·Ćū³Ę£ŗa £¬b ”£
£Ø2£©ÅØH2SO4ŠčŅŖĄäČ“ÖĮ0”ęŅŌĻĀµÄŌŅņŹĒ ”£
£Ø3£©·“Ó¦ŠčŅŖ½Į°č12h£¬ĘäŌŅņŹĒ ”£
£Ø4£©Č·¶Ø×īÖÕ²śĘ·ŹĒ4,7-¶ž¼×»łĻć¶¹ĖŲµÄŹµŃé»ņ·½·ØŹĒ ”£
£Ø5£©±¾“ĪŹµŃé²śĀŹĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com