
£Ø·Ö£©ĪļÖŹA~K¶¼ŹĒÓÉĻĀ±ķÖŠĄė×ÓŠĪ³ÉµÄ£¬ĒŅĪŖ֊ѧ»Æѧ³£¼ūĪļÖŹ£¬ÓŠČēĶ¼ĖłŹ¾µÄ×Ŗ»Æ¹ŲĻµ”£²æ·Ö²śĪļÖŠµÄĖ®ŅŃŹ”ĀŌ”£(Čē¹ūŠčŅŖŅõ”¢ŃōĄė×ÓæÉŅŌÖŲø“Ź¹ÓĆ)
ŅŃÖŖC”¢D”¢J¶¼ŹĒ°×É«³Įµķ£¬ĘäÖŠD”¢J²»ČÜÓŚĻ”ĻõĖį”£ŹŌ°“ŅŖĒó»Ų“šĻĀĮŠĻą¹ŲĪŹĢā£ŗ
£Ø1£©A”¢D”¢JµÄ»ÆѧŹ½ĪŖ£ŗA ”¢D ”¢J £»
£Ø2£©ĻąĶ¬Ģõ¼žĻĀ£¬ĪļÖŹµÄĮæÅضČĻąĶ¬µÄBČÜŅŗk*s#5^uŗĶEČÜŅŗpH½Ļ“óµÄŹĒ £ØÓĆ»ÆѧŹ½±ķŹ¾£©£»
£Ø3£©Š“³ö·“Ó¦¢ŪµÄĄė×Ó·½³ĢŹ½£ŗ
£Ø4£©ÓƶčŠŌµē¼«µē½āIČÜŅŗŃō¼«µÄµē¼«·“Ó¦·½³ĢŹ½ĪŖ£ŗ

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģøŹĖąŹ”ĢģĖ®ŹŠ¶žÖŠøßČżÄ£Äā£Ø5ŌĀ£©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
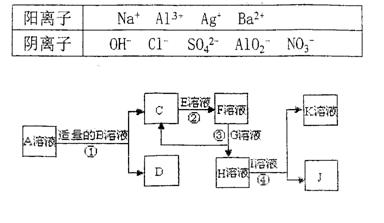
£Ø1£©ĢāĆææÕ1·Ö£¬ĘäÓąĆææÕ2·Ö£¬¹²12·Ö£©ĪļÖŹAŹĒ»ÆŗĻĪļ£¬B”¢C”¢D”¢K¶¼ŹĒµ„ÖŹ£¬·“Ó¦¢Ś”«¢Ż¶¼ŹĒ³£¼ūµÄ¹¤ŅµÉś²śµÄ·“Ó¦£¬ø÷ÓŠ¹ŲĪļÖŹÖ®¼äµÄĻą»„·“Ó¦×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£ŗ
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
(1)Š“³öĻĀĮŠĪļÖŹµÄ»ÆѧŹ½£ŗB £» C £» D £»
K £»G £»J ”£
(2)Š“³öĻĀĮŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
¢ŁH+E(ČÜŅŗ)”śM ”£
¢ŚIČÜÓŚG ”£
(3)ŌŚĶس£×“æöĻĀ£¬Čō1 g £ĆĘųĢåŌŚBĘųĢåÖŠČ¼ÉÕÉś³ÉHĘųĢåŹ±·Å³ö92.3 k£ŹČČĮ棬Ōņ2 mol HĘųĢåĶźČ«·Ö½āÉś³ÉCĘųĢåŗĶBĘųĢåµÄČČ»Æѧ·½³ĢŹ½ĪŖ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģÉĻŗ£ŹŠĖɽĒųøßČżÉĻѧʌʌĩ£Ø1ŌĀ£©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø±¾Ģā¹²8·Ö£©
NOŗĶCO¶¼ŹĒÓŠ¶¾µÄĘųĢ壬ĄūÓĆ“ß»Æ¼¼ŹõÄܹ»½«ĖüĆĒ×Ŗ±ä³ÉĪŽ¶¾µÄCO2ŗĶN2”£Ņ»¶ØĮæµÄNOŗĶCO½ųŠŠČēĻĀ·“Ó¦£ŗ2NO+2CO 2CO2+N2£¬Ęä²æ·Ö»ÆŃ§Ę½ŗā³£ŹżČēĻĀ±ķ£ŗ
2CO2+N2£¬Ęä²æ·Ö»ÆŃ§Ę½ŗā³£ŹżČēĻĀ±ķ£ŗ
| T£Ø”ę£© | 0 | 50 | 100 |
| K | 0.5 | 1.05 | 2.56 |
 CO2+1/2N2£¬Ōņ100”ꏱ£¬KµÄÖµ= ”£
CO2+1/2N2£¬Ōņ100”ꏱ£¬KµÄÖµ= ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğøŹĖąŹ”ĢģĖ®ŹŠøßČżÄ£Äā£Ø5ŌĀ£©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĶʶĻĢā
£Ø1£©ĢāĆææÕ1·Ö£¬ĘäÓąĆææÕ2·Ö£¬¹²12·Ö£©ĪļÖŹAŹĒ»ÆŗĻĪļ£¬B”¢C”¢D”¢K¶¼ŹĒµ„ÖŹ£¬·“Ó¦¢Ś”«¢Ż¶¼ŹĒ³£¼ūµÄ¹¤ŅµÉś²śµÄ·“Ó¦£¬ø÷ÓŠ¹ŲĪļÖŹÖ®¼äµÄĻą»„·“Ó¦×Ŗ»Æ¹ŲĻµČēĻĀĶ¼ĖłŹ¾£ŗ

ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
(1)Š“³öĻĀĮŠĪļÖŹµÄ»ÆѧŹ½£ŗB £» C £» D £»
K £»G £»J ”£
(2)Š“³öĻĀĮŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
¢ŁH+E(ČÜŅŗ)”śM ”£
¢ŚIČÜÓŚG ”£
(3)ŌŚĶس£×“æöĻĀ£¬Čō1 g £ĆĘųĢåŌŚBĘųĢåÖŠČ¼ÉÕÉś³ÉHĘųĢåŹ±·Å³ö92.3 k£ŹČČĮ棬Ōņ2 mol HĘųĢåĶźČ«·Ö½āÉś³ÉCĘųĢåŗĶBĘųĢåµÄČČ»Æѧ·½³ĢŹ½ĪŖ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÉĻŗ£ŹŠĖɽĒųøßČżÉĻѧʌʌĩ£Ø1ŌĀ£©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø±¾Ģā¹²8·Ö£©
NOŗĶCO¶¼ŹĒÓŠ¶¾µÄĘųĢ壬ĄūÓĆ“ß»Æ¼¼ŹõÄܹ»½«ĖüĆĒ×Ŗ±ä³ÉĪŽ¶¾µÄCO2ŗĶN2”£Ņ»¶ØĮæµÄNOŗĶCO½ųŠŠČēĻĀ·“Ó¦£ŗ2NO+2CO 2CO2+N2£¬Ęä²æ·Ö»ÆŃ§Ę½ŗā³£ŹżČēĻĀ±ķ£ŗ
2CO2+N2£¬Ęä²æ·Ö»ÆŃ§Ę½ŗā³£ŹżČēĻĀ±ķ£ŗ
|
T£Ø”ę£© |
0 |
50 |
100 |
|
K |
0.5 |
1.05 |
2.56 |
øł¾ŻĢāŅā»Ų“š£Ø¾ł²»æ¼ĀĒĪĀ¶Č±ä»Æ¶Ō“߻ƼĮ“߻Ɗ§ĀŹµÄÓ°Ļģ£©£ŗ
1£®Čō°Ń»Æѧ·½³ĢŹ½Š“ĪŖNO+CO CO2+1/2N2£¬Ōņ100”ꏱ£¬KµÄÖµ=
ӣ
CO2+1/2N2£¬Ōņ100”ꏱ£¬KµÄÖµ=
ӣ
2£®ÉĻŹö·“Ó¦“ļµ½»ÆŃ§Ę½ŗāŗó£¬ĻĀĮŠ“ėŹ©ÄÜĢįøßNO×Ŗ»ÆĀŹµÄŹĒ________”£
A£®Ń”ÓĆøüÓŠŠ§µÄ“߻ƼĮ ””””””””B£®Éżøß·“Ó¦ĢåĻµµÄĪĀ¶Č
C£®½µµĶ·“Ó¦ĢåĻµµÄĪĀ¶Č ””””””””D£®ĖõŠ”ČŻĘ÷µÄĢå»ż
3£®ČōÉĻŹö·“Ó¦ŌŚČŻ»ż²»±äµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ£¬øĆæÉÄę·“Ó¦“ļµ½Ę½ŗāµÄ±źÖ¾ŹĒ____
A£®ĘųĢåµÄĆÜ¶Č²»ŌŁ±ä»Æ
B£®µ„Ī»Ź±¼äÄŚĻūŗĵÄCOÓėÉś³ÉµÄCO2µÄĪļÖŹµÄĮæÖ®±ČĪŖ1©U1
C£®ĘųĢåµÄŃ¹Ēæ²»ŌŁ±ä»Æ
D£®ø÷ĘųĢåµÄÅضČĻąµČ
4£®Ä³ĪĀ¶ČĻĀ£¬½«0.02 molµÄNOŗĶ0.02 molµÄCOµÄ»ģŗĻĘųĢå³äČėŅ»×°ÓŠ“߻ƼĮµÄČŻĘ÷ÖŠ£¬³ä·Ö·“Ó¦ŗ󣬲āµĆ»ģŗĻĘųĢåÖŠCOµÄĢå»ż·ÖŹżĪŖ0.125£¬ŌņCOµÄ×Ŗ»ÆĀŹĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com