
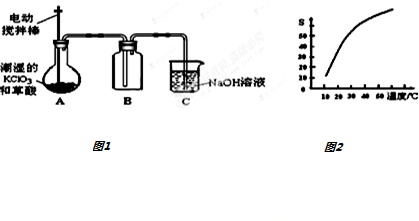
·ÖĪö £Ø1£©B×°ÖĆŹÕ¼ÆClO2£¬ĒŅČŪµćµĶ£¬Ó¦ÓĆĄäĖ®ĄäČ“ŹÕ¼Æ£»
£Ø2£©NaClO2Čܽā¶ČŹÕĪĀ¶ČÓ°Ļģ“ó£¬ŌŚĪĀ¶ČøßÓŚ38”ꏱĪö³ö¾§ĢåŹĒNaClO2£¬“ÓČÜŅŗÖŠ»ńµĆNaClO2¾§Ģ壬ŠčŅŖÕō·¢½į¾§”¢³ĆČČ»ņ38”ęŅŌÉĻ¹żĀĖ”¢Ļ“µÓ”¢øÉŌļµĆµ½£»
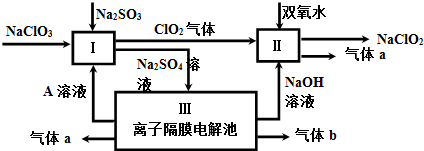
£Ø3£©¢ŁÓÉĮ÷³ĢĶ¼æÉÖŖ£¬¢óŹĒĄė×ÓøōĤµē½ā³Ų£¬µē½āĮņĖįÄĘČÜŅŗ±¾ÖŹŹĒµē½āĖ®£¬ĘųĢåaÓėČÜŅŗAŌŚĶ¬Ņ»µē¼«µĆµ½£¬NaOHÓėĘųĢåbŌŚĮķĶāµē¼«µĆµ½£¬¹ŹAĪŖĮņĖį£¬aĪŖŃõĘų£¬bĪŖĒāĘų£¬IÖŠNaClO3”¢Na2SO3”¢ĮņĖį·“Ӧɜ³ÉC1O2”¢Na2SO4ČÜŅŗ£¬·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖ£ŗ2H++SO32-+2ClO3-=2C1O2+SO42-+H2O£¬¢ņÖŠC1O2Óė¹żŃõ»ÆĒāŌŚ¼īŠŌĢõ¼žĻĀ·“Ӧɜ³ÉNaClO2ŗĶŃõĘų£»
¢ŚClO2æÉÓĆŃĒĀČĖįÄĘŗĶĻ”ŃĪĖįĪŖŌĮĻÖʱø£¬øł¾Żµē×Ó×ŖŅĘŹŲŗć£¬æÉÖŖ»¹ÓŠNaClÉś³É£»
¢Ū·¢Éś·“Ó¦£ŗ3NaClO2=2NaClO3+NaCl£¬3molNaClO2±äÖŹµĆµ½2mol NaClO3£¬Óė×ćĮæFeSO4ČÜŅŗ·“Ó¦Ź±£¬NaClO2”¢NaClO3¾ł±»Fe2+»¹ŌĪŖCl-£¬¼ĘĖć3molNaClO2”¢2mol NaClO3·“Ó¦»ńµĆµē×ÓŹżÄ森
½ā“š ½ā£ŗ£Ø1£©B×°ÖĆŹÕ¼ÆClO2£¬ĒŅČŪµćµĶ£¬Ó¦ÓĆĄäĖ®ĄäČ“ŹÕ¼Æ£¬Ź¹ClO2³ä·ÖĄäÄż£¬¼õÉŁ»Ó·¢£¬
¹Ź“š°øĪŖ£ŗŹ¹ClO2³ä·ÖĄäÄż£¬¼õÉŁ»Ó·¢£»
£Ø2£©NaClO2Čܽā¶ČŹÕĪĀ¶ČÓ°Ļģ“ó£¬ŌŚĪĀ¶ČøßÓŚ38”ꏱĪö³ö¾§ĢåŹĒNaClO2£¬“ÓČÜŅŗÖŠ»ńµĆNaClO2¾§Ģ壬ŠčŅŖÕō·¢½į¾§”¢³ĆČČ»ņ38”ęŅŌÉĻ¹żĀĖ”¢Ļ“µÓ”¢øÉŌļµĆµ½£¬
¹Ź“š°øĪŖ£ŗÕō·¢½į¾§”¢³ĆČČ»ņ38”ęŅŌÉĻ¹żĀĖ£»
£Ø3£©¢ŁÓÉĮ÷³ĢĶ¼æÉÖŖ£¬¢óŹĒĄė×ÓøōĤµē½ā³Ų£¬µē½āĮņĖįÄĘČÜŅŗ±¾ÖŹŹĒµē½āĖ®£¬ĘųĢåaÓėČÜŅŗAŌŚĶ¬Ņ»µē¼«µĆµ½£¬NaOHÓėĘųĢåbŌŚĮķĶāµē¼«µĆµ½£¬¹ŹAĪŖĮņĖį£¬aĪŖŃõĘų£¬bĪŖĒāĘų£¬IÖŠNaClO3”¢Na2SO3”¢ĮņĖį·“Ӧɜ³ÉC1O2”¢Na2SO4ČÜŅŗ£¬·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖ£ŗ2H++SO32-+2ClO3-=2C1O2+SO42-+H2O£¬¢ņÖŠC1O2Óė¹żŃõ»ÆĒāŌŚ¼īŠŌĢõ¼žĻĀ·“Ӧɜ³ÉNaClO2ŗĶŃõĘų£¬¢ņÖŠ·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖ£ŗ2ClO2+H2O2+2OH-=2ClO2-+O2”ü+2H2O£¬
¹Ź“š°øĪŖ£ŗ2ClO2+H2O2+2OH-=2ClO2-+O2”ü+2H2O£»
¢ŚClO2æÉÓĆŃĒĀČĖįÄĘŗĶĻ”ŃĪĖįĪŖŌĮĻÖʱø£¬øł¾Żµē×Ó×ŖŅĘŹŲŗć£¬æÉÖŖ»¹ÓŠNaClÉś³É£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗ5NaClO2+4HCl=5NaCl+4ClO2”ü+2H2O£¬
¹Ź“š°øĪŖ£ŗ5NaClO2+4HCl=5NaCl+4ClO2”ü+2H2O£»
¢Ū·¢Éś·“Ó¦£ŗ3NaClO2=2NaClO3+NaCl£¬3molNaClO2±äÖŹµĆµ½2mol NaClO3£¬Óė×ćĮæFeSO4ČÜŅŗ·“Ó¦Ź±£¬NaClO2”¢NaClO3¾ł±»Fe2+»¹ŌĪŖCl-£¬3molNaClO2·“Ó¦»ńµĆµē×ÓĪŖ3mol”Į4=12mol£¬2mol NaClO3·“Ó¦»ńµĆµē×ÓĪŖ2mol”Į6-12mol£¬¹ŹĻūŗÄFe2+ĪļÖŹµÄĮæĻąĶ¬£¬
¹Ź“š°øĪŖ£ŗĻąĶ¬£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹÖʱøŹµŃ飬ŹōÓŚĘ“ŗĻŠĶĢāÄ棬²ąÖŲ¶ŌÖʱøŌĄķÓėĪļÖŹµÄ·ÖĄėĢį“æµÄ漲飬½ĻŗƵÄæ¼²éѧɜ·ÖĪöĪŹĢā”¢½ā¾öĪŹĢāµÄÄÜĮ¦£¬ÄѶČÖŠµČ£®


 ³É¹¦ŃµĮ·¼Ę»®ĻµĮŠ“š°ø
³É¹¦ŃµĮ·¼Ę»®ĻµĮŠ“š°ø ±¶ĖŁŃµĮ··ØÖ±ĶØÖŠæ¼æ¼µćĻµĮŠ“š°ø
±¶ĖŁŃµĮ··ØÖ±ĶØÖŠæ¼æ¼µćĻµĮŠ“š°ø Ņ»¾ķøć¶ØĻµĮŠ“š°ø
Ņ»¾ķøć¶ØĻµĮŠ“š°ø ĆūŠ£×÷Ņµ±¾ĻµĮŠ“š°ø
ĆūŠ£×÷Ņµ±¾ĻµĮŠ“š°ø ĒįĒɶį¹ŚÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø
ĒįĒɶį¹ŚÖܲāŌĀæ¼Ö±ĶØĆūŠ£ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ·Å³öµÄČČĮæĪŖ£Ø0.4Q1+0.05Q3£©KJ | B£® | ·Å³öµÄČČĮæĪŖ£Ø0.4Q1+0.05Q2£©KJ | ||
| C£® | ”÷H2=”÷H3 | D£® | ”÷H2£¼”÷H3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
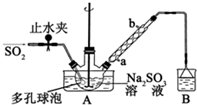 “ĪĮņĖįĒāÄĘ¼×Č©£ØNaHSO2•HCHO•2H2O£©Ė×³Ęµõ°×æ飬²»ĪČ¶Ø£¬120”ꏱ»į·Ö½ā£¬ŌŚÓ”Č¾”¢Ņ½Ņ©ŅŌ¼°Ō×ÓÄܹ¤ŅµÖŠÓŠ¹ć·ŗÓ¦ÓĆ£®ŅŌNa2SO3”¢SO2”¢HCHOŗĶŠæ·ŪĪŖŌĮĻÖʱø“ĪĮņĖįĒāÄĘ¼×Č©µÄŹµŃé²½ÖčČēĶ¼£ŗ
“ĪĮņĖįĒāÄĘ¼×Č©£ØNaHSO2•HCHO•2H2O£©Ė×³Ęµõ°×æ飬²»ĪČ¶Ø£¬120”ꏱ»į·Ö½ā£¬ŌŚÓ”Č¾”¢Ņ½Ņ©ŅŌ¼°Ō×ÓÄܹ¤ŅµÖŠÓŠ¹ć·ŗÓ¦ÓĆ£®ŅŌNa2SO3”¢SO2”¢HCHOŗĶŠæ·ŪĪŖŌĮĻÖʱø“ĪĮņĖįĒāÄĘ¼×Č©µÄŹµŃé²½ÖčČēĶ¼£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2 | B£® | 3 | C£® | 4 | D£® | 5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
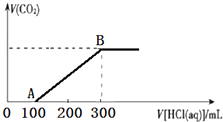 200mLijĪļÖŹµÄĮæÅØ¶ČµÄNaOHČÜŅŗÖŠ»ŗĀżĶØČėŅ»¶ØĮæµÄCO2£¬³ä·Ö·“Ó¦ŗó£¬µĆµ½Na2CO3ŗĶNaHCO3µÄ»ģŗĻČÜŅŗ£®ĻņÉĻŹöĖłµĆČÜŅŗÖŠ£¬ÖšµĪµĪ¼Ó2mol•L-1µÄŃĪĖį£¬ĖłµĆĘųĢåµÄĢå»żÓėĖł¼ÓŃĪĖįµÄĢå»ż¹ŲĻµČēĶ¼ĖłŹ¾£ŗ
200mLijĪļÖŹµÄĮæÅØ¶ČµÄNaOHČÜŅŗÖŠ»ŗĀżĶØČėŅ»¶ØĮæµÄCO2£¬³ä·Ö·“Ó¦ŗó£¬µĆµ½Na2CO3ŗĶNaHCO3µÄ»ģŗĻČÜŅŗ£®ĻņÉĻŹöĖłµĆČÜŅŗÖŠ£¬ÖšµĪµĪ¼Ó2mol•L-1µÄŃĪĖį£¬ĖłµĆĘųĢåµÄĢå»żÓėĖł¼ÓŃĪĖįµÄĢå»ż¹ŲĻµČēĶ¼ĖłŹ¾£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŖĖŲR Ī»ÓŚÖÜĘŚ±ķµÄIB ×壬ĘäŌ×ÓŠņŹżĪŖa£¬ŌņŌ×ÓŠņŹżĪŖa-3 µÄŌŖĖŲĪ»ÓŚ¢öB ×å | |
| B£® | ŌŚŌŖĖŲÖÜĘŚ±ķÖŠ 114 ŗÅŌŖĖŲµÄÉĻŅ»ÖÜĘŚĶ¬Ņ»×åŌŖĖŲµÄŌ×ÓŠņŹżŹĒ 82 | |
| C£® | ¾ßÓŠĻąĶ¬µē×Ó²ć½į¹¹µÄÖ÷×åŌŖĖŲĄė×ÓĪŖX2+”¢Y+£¬Ōņ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļµÄ¼īŠŌX£¾Y | |
| D£® | ŌŚÖÜĘŚ±ķÖŠ½šŹōÓė·Ē½šŹōµÄ·Ö½ēĻß“¦æÉŅŌÕŅµ½“߻ƼĮŗĶÄĶøßĪĀ”¢ÄĶøÆŹ“µÄŗĻ½š²ÄĮĻ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
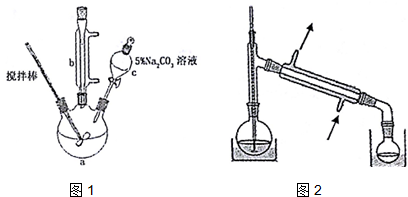
| ·Šµć | ČܽāŠŌ | ||
| ±ūĻ©Ėį | 141”ę | ÓėĖ®»„ČÜ£¬Ņ×ČÜÓŚÓŠ»śČܼĮ | ÓŠ¶¾ |
| ¼×“¼ | 65”ę | ÓėĖ®»„ČÜ£¬Ņ×ČÜÓŚÓŠ»śČܼĮ | Ņ×»Ó·¢£¬ÓŠ¶¾ |
| ±ūĻ©Ėį¼×õ„ | 80.5”ę | ÄŃČÜÓŚĖ®£¬Ņ×ČÜÓŚÓŠ»śČܼĮ | Ņ×»Ó·¢ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com