
 ������ͼ�ش����⣺
������ͼ�ش����⣺
| ||
| ||


 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
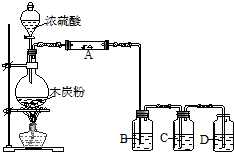
 ��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������
��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CCl4 |
| B��NO2 |
| C��PCl5 |
| D��NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
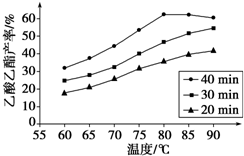
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
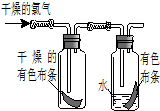 ��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����ĺ�ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽��������
��1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����ĺ�ʢ�г�ʪ��ɫ�����Ĺ��ƿ���ɹ۲쵽���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �������� | Cu��OH��2 | Fe��OH��3 | Fe��OH��2 | Mg��OH��2 |
| Ksp | 2.2��10-20 | 4.0��10-38 | 8.0��10-16 | 1.8��10-11 |
| �¶ȣ�K�� | ƽ��ʱNH3�����ʵ�����mol�� |
| T1 | 2.4 |
| T2 | 2.0 |
. |
| M |
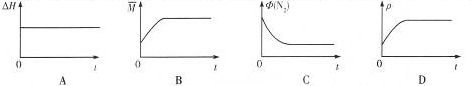
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Sc3+ |
| B��Mg2+ |
| C��Cl- |
| D��Br- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������� | B��̼���� |
| C���ƺ��� | D�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com