
 ̼�����ڲ�ͬ�¶��¿���ʧȥ���ֻ�ȫ���Ľᾧˮ������һ��̼���ƾ��壨Na2CO3•nH2O����Ʒ���ɲ����ڲ�ͬ�¶��¼�����Ʒ���¶������ߣ����ⶨ����ɣ�
̼�����ڲ�ͬ�¶��¿���ʧȥ���ֻ�ȫ���Ľᾧˮ������һ��̼���ƾ��壨Na2CO3•nH2O����Ʒ���ɲ����ڲ�ͬ�¶��¼�����Ʒ���¶������ߣ����ⶨ����ɣ�
| �������� | �����¶ȣ��棩 | ����+����������g�� |
| �� | ���� | 62.2 |
| �� | T1 | 56.8 |
| �� | T2 | 49.6 |
| �� | T3 | 44.2 |
| �� | T4 | 44.2 |
���� ��1���ٸ��ݳ������������IJ���������������ƽ����С���ȵ�λΪ0.1g��
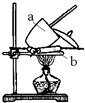
�ڸ���װ��ͼ���������չ����������������������������ϣ�
�����պ�Ҫ��ȴ�����£�Ȼ�������
�ܵ��������γ��������������0.1g��������Ϊ��������������ˣ����ᾧˮ��ȫʧȥ��
��Na2CO3•nH2O$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+nH2O�����������仯���ˮ����������Ͼ�������������ݷ���ʽ�������n��
��2��a���¶ȹ���������̼���Ʒֽ⣬�����ˮ������ƫ��
b��̼���ƾ�����Ʒ���������绯��������ˮƫ�٣������ˮ������ƫС��
c��̼���ƾ�����Ʒû����ȫʧȥ�ᾧˮ�������ˮ������ƫС��
d�����ȹ��������������彦���������ˮ������ƫ��
��� �⣺��1����ʵ����һ����������ƽ�����������������С���ȵ�λΪ0.1g��
�ʴ�Ϊ��������ƽ��0.1g��
�����չ�������������ͼ������aΪ��������a��ȷ�������������������ϣ���������bΪ�����ǣ�
�ʴ�Ϊ����ȷ�������ǣ�
�����պ�Ҫ��ȴ�����£����������С���������ָ��ȴ�����ڿ����к���ˮ�������ڿ�������ȴ����ˮ������Ҫ�ڸ���������ȴ��
�ʴ�Ϊ����ȴ����������
�ܵ��������γ��������������0.1g��������Ϊ��������������ˣ����ᾧˮ��ȫʧȥ�����������γ��������������0.1g����ֹͣʵ�飻
�ʴ�Ϊ���������γ��������������0.1g��
��m��H2O��=62.2-44.2=18g��m��Na2CO3��=44.2-33.6=10.6g
Na2CO3•nH2O$\frac{\underline{\;\;��\;\;}}{\;}$Na2CO3+nH2O
106 18n
10.6g 18g
����n=10��
�ʴ�Ϊ��10��
��2��a���¶ȹ���������̼���Ʒֽ⣬�����ˮ������ƫ���������nƫ��a��ѡ��
b��̼���ƾ�����Ʒ���������绯��������ˮƫ�٣������ˮ������ƫС�����������nƫС����bѡ��
c��̼���ƾ�����Ʒû����ȫʧȥ�ᾧˮ�������ˮ������ƫС�����������nƫС����cѡ��
d�����ȹ��������������彦���������ˮ������ƫ���������nƫ��d��ѡ��
�ʴ�Ϊ��bc��
���� ���⿼����������ɵIJⶨ��������ʵ����������ݴ�����֪ʶ�Ŀ��飬��Ŀ�Ѷ��еȣ�������ѧ����ʵ��̽�����������ݴ���������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 CH3COOC2H5+H2O������˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬����ad��
CH3COOC2H5+H2O������˵���÷�Ӧ�Ѿ��ﵽ��ѧƽ��״̬����ad���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | W��Y��Z �ĵ縺�Դ�С˳��һ����Z��Y��W | |
| B�� | Z ���⻯���֮�������� | |
| C�� | Y��Z �γɵķ��ӵ�����ԭ�ӿ��ܲ���sp3�ӻ� | |
| D�� | WY2�����ЦҼ���м�����Ŀ֮����2��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ҫͨ����ѧ��Ӧ���ܴӺ�ˮ�л��ʳ�κ͵�ˮ | |
| B�� | DZˮͧ�ڽ�������¿��ù������ƹ��� | |
| C�� | �������ռ���Һ��ʯ���鷴Ӧ���ܵõ����������� | |
| D�� | ��ҵ�ϳ��ù�������ά�������ۻ�ԭһЩ�������������ƽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | X������������Ӧˮ��������Ա�Y������������Ӧˮ���������ǿ | |
| B�� | Xԭ�ӵĵõ���������Yԭ�ӵĵõ�������ǿ | |
| C�� | X�������ӵĻ�ԭ�Ա�Y�������ӵĻ�ԭ��ǿ | |
| D�� | X����̬�⻯���Y����̬�⻯���ȶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
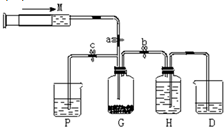 ij����С��������ͼ��ʾװ����ȡ�������ṩ���Լ��У�Ũ���ᡢ����ʳ��ˮ������������Һ��������ع��壮��Ӧ�Ļ�ѧ����ʽΪ��2KMnO4+16HCl��Ũ��=2KCl+2MnCl2+5Cl2��+8H2O
ij����С��������ͼ��ʾװ����ȡ�������ṩ���Լ��У�Ũ���ᡢ����ʳ��ˮ������������Һ��������ع��壮��Ӧ�Ļ�ѧ����ʽΪ��2KMnO4+16HCl��Ũ��=2KCl+2MnCl2+5Cl2��+8H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO3+H2O��H2SO4 | B�� | Cl2+H2O?HCl+HClO | ||
| C�� | 2 F2+2 H2O��4 HF+O2 | D�� | 2 Al+6 H2O$\stackrel{��}{��}$2 Al��OH��3+3 H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ϵ�������Ա������е��ؽ�����Ⱦ | |
| B�� | �������������ȶ������ά��ǿ��ᡰ��·�� | |
| C�� | ͨ�����ع��͡����Ի�����ͣ����Ϊ�� | |
| D�� | ����ȥ�����۵���Ч�ɷ��Ǹ�֬������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2mol KClO3 | B�� | 1mol KCl | C�� | 1mol Ca��ClO��2 | D�� | 2mol NaCl |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com