
£Ø16·Ö£©øÖĢśĘóŅµĖįĻ“øÖ²ÄŹ±²śÉśµÄ·ĻŅŗÖ÷ŅŖ³É·ÖĪŖFe2+”¢H+”¢Cl-£¬æÉÓĆĻĀŹö·½·Ø“¦ĄķøĆ·ĻŅŗ£¬»ŲŹÕŃĪĖįŗĶÖʱøŃõ»ÆĢśĶæĮĻ”£ 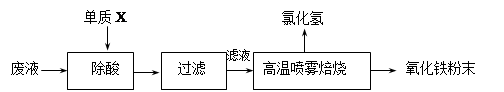
£Ø1£©µ„ÖŹXµÄ»ÆѧŹ½ŹĒ ”£
£Ø2£©ĀČ»ÆŃĒĢśČÜŅŗ¾øßĪĀÅēĪķ±ŗÉÕ×Ŗ»ÆĪŖHClĘųĢåŗĶŃõ»ÆĢś·ŪÄ©£¬ÓŠ¹ŲµÄ»Æѧ·½³ĢŹ½ŅĄ“ĪĪŖ£ŗ ”£
£Ø3£©Ä³ĢśŗģĶæĮĻÖŠ³żŗ¬ÓŠFe2O3Ķā£¬»¹æÉÄÜĢķ¼ÓÓŠCuO»ņFeOÖŠµÄŅ»ÖÖ,ĒėÉč¼ĘŹµŃé·½°ø£¬Ģ½¾æøĆĢśŗģĶæĮĻÖŠĢķ¼ÓĪļµÄ³É·Ö”£
¢Ł Ģį³öŗĻĄķ¼ŁÉč
¼ŁÉč1£ŗĢķ¼ÓĮĖCuO
¼ŁÉč2£ŗĢķ¼ÓĮĖFeO
¢Ś ĒėÉč¼Ę·½°ø£¬ŃéÖ¤ÉĻŹö¼ŁÉč£¬Š“³öŹµŃé²½Öč”¢Ō¤ĘŚĻÖĻóŗĶ½įĀŪ”£
ĻŽŃ”ŹŌ¼Į£ŗĢś·Ū”¢3mol?L-1H2SO4”¢0.1 mol?L-1ĖįŠŌKMnO4ČÜŅŗ”¢10%NaOHČÜŅŗ”¢10%H2O2”¢KSCNČÜŅŗ
| ²Ł×÷²½Öč | Ō¤ĘŚĻÖĻóŗĶ½įĀŪ |
| ²½Öč1£®Č”ÉŁĮæѳʷӌŹŌ¹ÜÖŠ£¬ ____________________________________________ | ѳʷȫ²æČܽā£¬µĆµ½³ĪĒåµÄČÜŅŗ”£ |
| ²½Öč2£® ²½Öč3£® | ___________________________ ___________________________ |
£Ø16·Ö£©£Ø1£© Fe £Ø1·Ö£¬Š“Ćū³Ę²»øų·Ö£©
£Ø2£©4FeCl2+ 4H2O+ O2  2Fe2O3 + 4HCl£Ø3·Ö£©
2Fe2O3 + 4HCl£Ø3·Ö£©
£Ø»ņ FeCl2+ 2H2O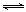 Fe(OH)2+ 2HCl£»4Fe(OH)2+O2+2H2O = 4Fe(OH)3£»
Fe(OH)2+ 2HCl£»4Fe(OH)2+O2+2H2O = 4Fe(OH)3£»
2Fe(OH)3 Fe2O3+ 3H2O£¬ĆæŹ½1·Ö£¬¹²3·Ö”£Ģõ¼ž¼°æÉÄę·ūŗŲ»×÷ĪŖµĆ·Öµć£©
Fe2O3+ 3H2O£¬ĆæŹ½1·Ö£¬¹²3·Ö”£Ģõ¼ž¼°æÉÄę·ūŗŲ»×÷ĪŖµĆ·Öµć£©
£Ø3£©£Ø10·Ö£©
£Ø4£© 224 £Ø2·Ö£©²Ł×÷²½Öč Ō¤ĘŚĻÖĻóŗĶ½įĀŪ ²½Öč1£®Č”ÉŁĮæѳʷӌŹŌ¹ÜÖŠ£¬¼ÓČė×ćĮæµÄ3mol?L£1H2SO4£¬³ä·ÖÕńµ“”££Ø2·Ö£© ²½Öč2£®Č”ÉŁŠķÉĻŹöČÜŅŗÓŚ£¬¼ÓČė×ćĮæĢś·Ū£¬³ä·ÖÕńµ“£»ŌŁ¼ÓČė×ćĮæ3mol?L£1H2SO4£¬³ä·ÖÕńµ“”££Ø2·Ö£©
²½Öč3£®Č”ÉŁŠķ²½Öč1ČÜŅŗÓŚŹŌ¹ÜÖŠ£¬ÖšµĪ¼ÓČė0.01 mol?L£1ĖįŠŌKMnO4ČÜŅŗ.£Ø2·Ö£©
£Ø²½Öč2ŗĶ²½Öč3µÄ²Ł×÷ŗĶĻÖĻó½įĀŪæÉŅŌ»„»»Ī»ÖĆ£© ČōŹŌ¹ÜÖŠ³öĻÖŗģÉ«¹ĢĢ壬ĖµĆ÷ŹŌŃłÖŠÓŠCuO£Ø2·Ö£©
Čē¹ū×ĻŗģÉ«ĶŹČ„£¬ĖµĆ÷ŹŌŃłÖŠÓŠFeO£Ø2·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©øł¾ŻĮ÷³ĢµÄÄæµÄæÉÖŖ£¬XŹĒFe£¬ÓėĖį·“Ӧɜ³ÉŃĒĢśĄė×Ó£¬æɳżČ„ĖįĶ¬Ź±²»ŅżČėĘäĖūŌÓÖŹ£»
£Ø2£©ĀČ»ÆŃĒĢśČÜŅŗ¾øßĪĀÅēĪķ±ŗÉÕ×Ŗ»ÆĪŖHClĘųĢåŗĶŃõ»ÆĢś·ŪÄ©£¬æÉÖŖøĆ¹ż³Ģ·¢ÉśĮĖŃõ»Æ»¹Ō·“Ó¦£¬ĖłŅŌÓ¦ÓŠŃõĘų²Ī¼Ó£¬øßĪĀĻąµ±ÓŚøųĢåĻµ¼ÓČČ£¬Ńõ»ÆĢśµÄÉś³ÉÓ¦Ą“×ŌŃĒĢśĄė×ÓµÄĖ®½ā£¬Éś³ÉĒāŃõ»ÆŃĒĢś£¬ĒāŃõ»ÆŃĒĢś±»ŃõĘųŃõ»ÆµĆĒāŃõ»ÆĢś£¬ĒāŃõ»ÆĢś·Ö½ā²śÉśŃõ»ÆĢś£¬ĖłŅŌøĆ¹ż³ĢÖŠÉę¼°µÄ»Æѧ·½³ĢŹ½ÓŠ£ŗFeCl2+ 2H2O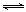 Fe(OH)2+ 2HCl£»4Fe(OH)2+O2+2H2O = 4Fe(OH)3£»2Fe(OH)3
Fe(OH)2+ 2HCl£»4Fe(OH)2+O2+2H2O = 4Fe(OH)3£»2Fe(OH)3 Fe2O3+ 3H2O£»
Fe2O3+ 3H2O£»
£Ø3£©øł¾Ż±ķÖŠĖłøųµÄŌ¤ĘŚĻÖĻóŗĶ½įĀŪ£¬²½Öč1ӦєŌńĖį£¬ŅņĪŖĪŽĀŪŹĒCuO»¹ŹĒFeO¶¼ÄÜČÜÓŚĖį£¬ĖłŅŌ²Ł×÷1ĪŖ£ŗ¼ÓČė×ćĮæµÄ3mol?L£1H2SO4£¬³ä·ÖÕńµ“£»“ĖŹ±ČÜŅŗÖŠŗ¬ÓŠCu2+”¢Fe2+”¢Fe3+£¬²½Öč2”¢3ŌņÓ¦ŹĒ¼ģŃéČÜŅŗÖŠµÄCu2+»ņFe2+£¬øł¾ŻĖłøųŹŌ¼Į£¬¼ģŃéCu2+£¬Ó¦Ń”ŌńĢś·Ū”¢3mol?L-1H2SO4£¬¼ģŃéFe2+£¬Ó¦ÓĆ0.1 mol?L-1ĖįŠŌKMnO4ČÜŅŗ£¬¾ßĢå²½ÖčĻź¼ū“š°ø£»
£Ø4£©ÉčÓĆ635gĖ®ĶźČ«ĪüŹÕ”°øßĪĀÅēĪķ±ŗÉÕ”±²śÉśµÄHClĘųĢåxmolµĆµ½36.5%µÄÅØŃĪĖį£¬Ōņ36.5x/(635+36.5x)=36.5%£¬½āµĆx=10£¬ĖłŅŌæÉĪüŹÕ±ź×¼×“æöĻĀµÄHClµÄĢå»żŹĒ224LæɵĆ36.5%µÄÅØŃĪĖį”£
æ¼µć£ŗæ¼²é¶Ō¹¤ŅµĮ÷³ĢµÄ·ÖĪöÓė¼ĘĖć£¬Ąė×ӵļģŃ飬ŹµŃé·½°øµÄÉč¼Ę



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ÓŠĻąĶ¬ÖŹĮæµÄĮ½·ŻNaHCO3·ŪÄ©£¬µŚŅ»·Ż¼ÓČė×ćĮæŃĪĖį£¬óŹ¶ž·ŻĻČ¼ÓČČŹ¹ĘäĶźČ«·Ö½ā
ŌŁ¼Ó×ćĮæĶ¬ÖŹĮæ·ÖŹżµÄŃĪĖį£¬ŌņĮ½ÕßĖłĻūŗĵÄŃĪĖįÖŠĀČ»ÆĒāµÄÖŹĮæ±ČĪŖ
| A£®2:1 | B£®1:1 | C£®1:2 | D£®4:2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
Al”¢Fe”¢Cu¶¼ŹĒÖŲŅŖµÄ½šŹōŌŖĖŲ”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
| A£®ČżÕ߶ŌÓ¦µÄŃõ»ÆĪļ¾łĪŖ¼īŠŌŃõ»ÆĪļ |
| B£®ČżÕߵĵ„ÖŹ·ÅÖĆŌŚæÕĘųÖŠ¾łÖ»Éś³ÉŃõ»ÆĪļ |
C£®ÖʱøAlCl3”¢Fe Cl3”¢CuCl2¾ł²»ÄܲÉÓĆ½«ČÜŅŗÖ±½ÓÕōøɵķ½·Ø Cl3”¢CuCl2¾ł²»ÄܲÉÓĆ½«ČÜŅŗÖ±½ÓÕōøɵķ½·Ø |
| D£®AlCl3”¢FeCl3”¢CuCl2¾łæÉÓĆ×÷¾»Ė®¼Į |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
½«ag Mg”¢AlŗĻ½šĶźČ«ČܽāŌŚV1L”¢c1mol/LµÄŃĪĖįČÜŅŗÖŠ£¬²śÉśbgH2”£ŌŁĻņ·“Ó¦ŗóµÄČÜŅŗÖŠ¼ÓČėV2L,c2mol/LNaOHČÜŅŗ£¬Ē”ŗĆŹ¹³Įµķ“ļµ½×ī“óÖµ£¬ĒŅ³ĮµķÖŹĮæĪŖdg”£ŌņĻĀĮŠ¹ŲĻµŹ½“ķĪóµÄŹĒ( )
| A£®ŗĻ½šÖŠµÄĪļÖŹµÄĮæĪŖ(24b-a)/9mol |
| B£®d=a+17b |
C£®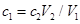 |
D£®Óė½šŹō·“Ó¦ŗóŹ£ÓąŃĪĖįµÄĪļÖŹµÄĮæÅضČĪŖ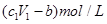 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĪŖĮĖ²ā¶ØĒāŃõ»ÆÄĘŗĶĢ¼ĖįÄĘ¹ĢĢå»ģŗĻĪļÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬¼×”¢ŅŅĮ½Ī»Ķ¬Ń§·Ö±šÉč¼ĘĮĖČēĻĀµÄŹµŃé·½°ø£ŗ
(I)¼×Ķ¬Ń§µÄ·½°øŹĒ£ŗ½«mgѳʷČܽā£¬¼Ó¹żĮæĀČ»Æ±µČÜŅŗ£¬¹żĀĖ”¢Ļ“µÓ”¢ŗęøÉ£¬³ĘµĆ¹ĢĢång”£
£Ø1£©»ģŗĻĪļÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹżĪŖ(ÓĆm”¢n±ķŹ¾) £¬¼×Ķ¬Ń§Ļ“µÓ³ĮµķµÄ¾ßĢå²Ł×÷ŹĒ ”£
£Ø2£©Ca2+”¢Ba2+¶¼æÉŅŌŹ¹CO32-³ĮµķĶźČ«£¬µ«Ź¹ÓĆĀČ»Æ±µČÜŅŗ±ČĀČ»ÆøĘČÜŅŗĖłµĆµÄ½į¹ū¾ßÓŠøüøߵľ«Č·¶Č£¬ŌŅņŹĒ£ŗ¢Ł ;
¢Ś ”£
(¢ņ)ŅŅĶ¬Ń§µÄ·½°øČēĶ¼ĖłŹ¾£ŗ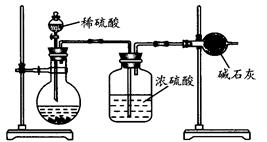
£Ø1£©øł¾ŻŅŅĶ¬Ń§µÄŹµŃé×°ÖĆĶ¼·ÖĪö£¬ŌŚĆæ“ĪŹµŃéÖŠ£¬Ķź³É²ā¶Ø¹ż³ĢÖĮÉŁŅŖ½ųŠŠ “Ī³ĘĮæ²Ł×÷”£°“ÕÕøĆĶ¬Ń§µÄ·½°ø½ųŠŠ²ā¶Ø£¬½į¹ūÓėŹµ¼ŹĒéæö“ęŌŚ½Ļ“óµÄĪó²ī£¬ÄćČĻĪŖÖ÷ŅŖŌŅņæÉÄÜŹĒ(ČĪŠ“Į½øö)£ŗ
¢Ł £»
¢Ś ”£
£Ø2£©ŅŅĶ¬Ń§µÄ·½°øµÄ×°ÖĆÖŠ“ęŌŚŅ»¶ØµÄȱĻŻ£¬ĒėÄćĢį³öøĽųµÄ·½·Ø(¼ņµ„ŠšŹö×ö·Ø£¬²»±Ų»Ķ¼)£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŚĻĀĶ¼ĖłŹ¾µÄĪļÖŹ×Ŗ»Æ¹ŲĻµÖŠ£Ø·“Ó¦Ģõ¼žŗĶ²æ·ÖÉś³ÉĪļĪ“Č«²æĮŠ³ö£©£¬XĪļÖŹæÉŅŌĄ“×ŌÓŚŗ£ŃóÖŠ£¬A”¢BĪŖ³£¼ūĘųĢåµ„ÖŹ£¬BĪŖ»ĘĀĢÉ«ĘųĢ壬I”¢LĪŖ³£¼ūµÄ½šŹōµ„ÖŹ£¬GĪŖŗģŗÖÉ«ĪļÖŹ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©X»ÆѧŹ½ĪŖ ”£ £Ø2£©C»ÆѧŹ½ĪŖ ”£
£Ø3£©·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø4£©·“Ó¦¢ŚµÄĄė×Ó·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©ŅŃÖŖ£ŗAĪŖŗ¬½šŹōĄė×ӵĵ»ĘÉ«¹ĢĢå»ÆĢØĪļ£¬E”¢XĪŖæÕĘųÖŠ³£¼ūĘųĢ壬A”¢B”¢C”¢Dŗ¬ÓŠĻąĶ¬µÄ½šŹōĄė×Ó£¬Ęä×Ŗ»Æ¹ŲĻµČēĻĀĶ¼£Ø²æ·Ö²śĪļŅŃĀŌČ„£©”£
Ēė»Ų“šĻĀĮŠĪŹĢā
£Ø1£©ÕāÖÖ½šŹōĄė×ӵĥė×Ó½į¹¹Ź¾ŅāĶ¼ĪŖ_____________;
£Ø2£©XµÄµē×ÓŹ½_______________;
£Ø3£©BÖŠĖłŗ¬»Æѧ¼üµÄĄąŠĶŹĒ_____________;
³£ĪĀ³£Ń¹ĻĀ£¬7.8gAÓė×ćĮæµÄĖ®³ä·Ö·“Ó¦·Å³öČČĮæa kJ£¬Š“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½
_________________________________________________________________.
£Ø4£©¢ŁCŅ²æÉ×Ŗ»ÆĪŖB£¬Š“³öøĆ×Ŗ»ÆµÄ»Æѧ·½³ĢŹ½_______________________________;
¢ŚBÓėD·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ_______________________________________.
£Ø5£©½«Ņ»¶ØĮæµÄĘųĢåXĶØČė2LBµÄČÜŅŗÖŠ£¬ĻņĖłµĆČÜŅŗÖŠ±ßÖšµĪ¼ÓČėĻ”ŃĪĖį±ßÕńµ“ÖĮ¹żĮ棬²śÉśµÄĘųĢåÓėŃĪĖįĪļÖŹµÄĮæµÄ¹ŲĻµČēĶ¼£ØŗöĀŌĘųĢåµÄČܽāŗĶHClµÄ»Ó·¢£©”£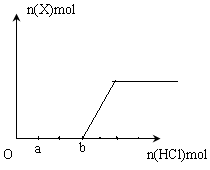
Ēė»Ų“š£ŗaµćČÜŅŗÖŠĖłŗ¬ČÜÖŹµÄ»ÆѧŹ½ĪŖ__________ £¬a£bÖ®¼äµÄ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©ŃĢĘųĶŃĮņÄÜÓŠŠ§¼õÉŁ¶žŃõ»ÆĮņµÄÅÅ·Å”£ŹµŃéŹŅÓĆ·ŪĆŗ»Ņ£ØÖ÷ŅŖŗ¬Al2O3”¢SiO2µČ£©Öʱø¼īŹ½ĮņĖįĀĮ[Al2(SO4)x(OH)6”Ŗ2x]ČÜŅŗ£¬²¢ÓĆÓŚŃĢĘųĶŃĮņŃŠ¾æ”£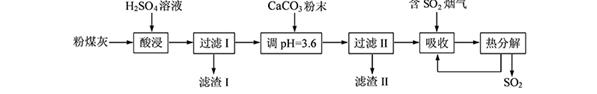
£Ø1£©Ėį½žŹ±·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £»ĀĖŌü¢ńµÄÖ÷ŅŖ³É·ÖĪŖ £ØĢī»ÆѧŹ½£©”£
£Ø2£©¼ÓCaCO3µ÷½ŚČÜŅŗµÄpHÖĮ3.6£¬ĘäÄæµÄŹĒÖŠŗĶČÜŅŗÖŠµÄĖį£¬²¢Ź¹Al2(SO4)3×Ŗ»ÆĪŖAl2(SO4)x(OH)6”Ŗ2x”£ĀĖŌü¢ņµÄÖ÷ŅŖ³É·ÖĪŖ £ØĢī»ÆѧŹ½£©£»ČōČÜŅŗµÄpHĘ«øߣ¬½«»įµ¼ÖĀČÜŅŗÖŠĀĮŌŖĖŲµÄŗ¬Įæ½µµĶ£¬ĘäŌŅņŹĒ £ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©”£
£Ø3£©ÉĻŹöĮ÷³ĢÖŠ¾ĶźČ«ČČ·Ö½ā·Å³öµÄSO2Įæ×ÜŹĒŠ”ÓŚĪüŹÕµÄSO2Į棬ĘäÖ÷ŅŖŌŅņŹĒ £»ÓėĪüŹÕSO2Ē°µÄČÜŅŗĻą±Č£¬ČČ·Ö½āŗóŃ»·ĄūÓƵÄČÜŅŗµÄpH½« £ØĢī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĢśŠ¼æÉÓĆÓŚµŲĻĀĖ®ĶѵŖ£¬Ę䏵ŃéŹŅŃŠ¾æ²½ÖčČēĻĀ£ŗ
¢ń”¢½«ĢśŠ¼½žÅŻŌŚ0.5 mol/LŃĪĖįÖŠ½ųŠŠŌ¤“¦Ąķ”£
¢ņ”¢30 minŗó£¬ÓĆČ„Ąė×ÓĖ®·“ø“³åĻ“£¬ÖĮ³åĻ“ŗóŅŗĢåµÄpHĪŖÖŠŠŌ”£ŌŚN2±£»¤ĻĀŗęøɱøÓĆ”£
¢ó”¢ŌŚÕōĮóĖ®ÖŠ¼ÓČėĻõĖį¼ŲÅäÖĘĻõĖį¼ŲČÜŅŗ”£
¢ō”¢½«ĖįŌ¤“¦ĄķŗóµÄĢśŠ¼¼ÓČėĻõĖį¼ŲČÜŅŗÖŠ”£
Ēė»Ų“š£ŗ
£Ø1£©ŃĪĖįČܽāFe2O3µÄĄė×Ó·½³ĢŹ½ŹĒ ”£
£Ø2£©ŗęøÉŹ±ŠčŅŖŌŚN2±£»¤ĻĀ½ųŠŠµÄŌŅņŹĒ ”£
£Ø3£©½«²½Öč¢ņÖŠ³åĻ“ŗóµÄČÜŅŗŌŚæÕĘųÖŠ¼ÓČČÕō·¢×ĘÉÕ£¬×īÖÕµĆµ½µÄ¹ĢĢåŹĒ ”£
£Ø4£©½«ĖįŠŌĢõ¼žĻĀ£¬ĢśÓėNO3£·“Ó¦µÄĄė×Ó·½³ĢŹ½²¹³äĶźÕū£ŗ
£Ø5£©ŅŃÖŖ»īŠŌĢæÄÜĪüø½NH4£« ”¢OH£”£²½Öč¢ōÖŠ²Ł×÷Ź±½«ĢśŠ¼ŗĶ»īŠŌĢæĶ¬Ź±¼ÓČėĻõĖį¼ŲČÜŅŗÖŠ£¬æÉŅŌĢįøßĶѵŖµÄŠ§¹ū£¬ĘäŌŅņŹĒ ”£
£Ø6£©ŃŠ¾æ±ķĆ÷ČÜŅŗpH»įÓ°ĻģĢśŠ¼ĶѵŖµÄŠ§¹ū£¬·“Ó¦ĢåĻµµÄpH·Ö±šæŲÖĘŌŚ4 ŗĶ8. 5 Ź±£¬NO3£µÄČ„³żĀŹ·Ö±šĪŖ90% ŗĶ15%”£Õż³£µŲĻĀĖ®ÖŠŗ¬ÓŠCO32££¬»įÓ°ĻģĶѵŖµÄŠ§¹ū£¬ÓĆ»ÆѧÓĆÓļŗĶĪÄ×Ö¼ņŹöĘäŌŅņ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com