
| |||||||||||

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ��˳�� | ʵ������ | ʵ������ |
| �� | A+B | ���������� |
| �� | B+D | ����ɫ��ζ����ų� |
| �� | C+B | �а�ɫ�������� |
| �� | A+D | �а�ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���½���³ľ�����2010��2011ѧ���һ��ѧ�����п��Ի�ѧ���� ���ͣ�022
Ϊ
��ȷ����ƿʧȥ��ǩ����ɫ��Һ�����Ƿֱ���ϡ���ᡢϡ���ᡢ����������̼������Һ��ijͬѧ������������ϵķ��������ܽ���ƿ��Һ�ֱ���ΪA��B��C��D��Ȼ���ȡ�������Թ���������ϣ����۲쵽�������������ʾ(��������ʾ����������������ʾ�г������ɣ���������ʾ���������ɣ�)��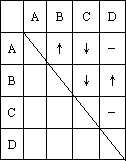
(1)�������ʵĻ�ѧʽ�ֱ��ǣ�
A________��B________��C________��D________
(2)д���йط�Ӧ�����ӷ���ʽ��
A��B��________
C��D��________
A��C��________
B��C��________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ѧ����ñ���һ��ѧ�˽̰� �˽̰� ���ͣ�022
Ϊ��ȷ����ƿʧȥ��ǩ����ɫ��Һ�����Ƿֱ���ϡ���ᡢ�������ơ�����������̼������Һ��ijͬѧ������������ϵķ�����������ƿ��Һ�ֱ���ΪA��B��C��D��Ȼ���ȡ�������Թ���������ϣ����۲쵽�������������ʾ(��������ʾ����������������ʾ�г������ɣ���������ʾ����������)
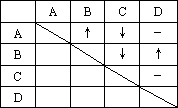
(1)�������ʵĻ�ѧʽ�ֱ��ǣ�
A��_____________________��
B��_____________________��
C��_____________________��
D��_____________________��
(2)д���йط�Ӧ�����ӷ���ʽ��
A��B��_____________________��
C��D��_____________________��
A��C��_____________________��
B��C��_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com