
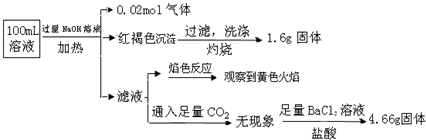
| ѡ�� | ��Ⱦ�� | ������ʩ | ������� |
| A | ���Եķ�ˮ | ��CO2�к� | ��ѧ�� |
| B | Cu2+���ؽ������� | �������γ��� | ��ѧ�� |
| C | �������л���ķ�ˮ | ͨ�������л | ������ |
| D | ���� | ����ʯ���к� | ��ѧ�� |
���� 1��I��ȡ������Һ������KSCN��Һʱ�����Ա仯��֤����Һ�в��������ӣ�
����ȡ��Һ�������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��˵����һ����������Ϊ����������֤��ԭ��Һ��һ������NO3-��Fe2+��������CO32-��SiO32-��
�������������Һ�м���BaCl2��Һ���а�ɫ�������ɣ�ȡԭ��Һ����BaCl2��Һ���а�ɫ�������ɣ�֤����Һ����SO42-��
���������������Һ�м������Ũ��ˮ�����������������ɫ��������Һ��û��Al3+�����ˣ���������Һ�м�������������ʱ���ټ�������������Һ������ɫ�������ɣ�������������ͭ����������Һ�к���Cu2+��
��Ⱦ����Ҫ���ܳ����ж������ʣ������ɵ����ʶԻ�������Ⱦ���������뻯ѧ���������Ƿ������������ɣ��ݴ˷�����
��� �⣺I��ȡ������Һ������KSCN��Һʱ�����Ա仯��֤����Һ�в��������ӣ�
����ȡ��Һ�������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��˵����һ����������Ϊ����������֤��ԭ��Һ��һ������NO3-��Fe2+��������CO32-��SiO32-��
�������������Һ�м���BaCl2��Һ���а�ɫ�������ɣ�ȡԭ��Һ����BaCl2��Һ���а�ɫ�������ɣ�֤����Һ����SO42-��
���������������Һ�м������Ũ��ˮ�����������������ɫ��������Һ��û��Al3+�����ˣ���������Һ�м�������������ʱ���ټ�������������Һ������ɫ�������ɣ�������������ͭ����������Һ�к���Cu2+��
��1�����Ͽ�֪���÷�ˮһ�����е�������Fe2+��Cu2+��NO3-��SO42-���ʴ�Ϊ��Fe2+��Cu2+��NO3-��SO42-��
��2��ȡ��Һ�������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��˵����һ����������Ϊ�������������������������ӷ�Ӧ����NO�����������ӡ�ˮ�������ӷ���ʽΪ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
�ʴ�Ϊ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
��3����Ӧ���������͵����������Ϊ1��4������ƽ����ʽΪ��16Al+9NO3-+7OH-=16AlO2-+4N2��+NH3��+2H2O�����ϼ۽��͵�������Ϊ��ԭ�����NH3��N2������֪0.2molNO3-��
16Al+9NO3-+7OH-=16AlO2-+4N2��+NH3��+2H2O
16 9
n��Al�� 0.2mol
n��Al��=$\frac{3.2}{9}$mol
��Al������Ϊ $\frac{3.2}{9}$mol��27 g•mol-1=9.6g��
�ʴ�Ϊ��NH3��N2��9.6��
��4����Ⱦ����Ҫ���ܳ����ж������ʣ������ɵ����ʶԻ�������Ⱦ���������뻯ѧ���������Ƿ������������ɣ�
A�����Եķ�ˮ�������̼��Ӧ���ɵ�̼���κ�ˮ������Ⱦ����A��ȷ��
B������ͭ���ܣ����Բ��ܳ�����ȥ��Ӧѡ�����������ͭ��������B����
C��������ˮ�еı�ͨ�������л�����ǻ�ѧ��������C����
D����ͼ���кͷ�ӦΪ��ѧ��Ӧ��Ϊ��ѧ���������Ƶ�ˮ��Һ�Լ��ԣ���D��ȷ��
��ѡAD��
���� ���⿼�������Ӽ����ʵ�鷽���ͷ�Ӧ������������ӷ���ʽ����д�������������ʺ���Һ�е���غ�����жϴ��ڵ������ǽ���ؼ�����Ŀ�ѶȽϴ�


 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д� Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����¶ȿ��Լӿ췴Ӧ���� | |
| B�� | ʹ�ú��ʵĴ������Լӿ췴Ӧ������ | |
| C�� | �����������£�������ȫ��ת��Ϊ���� | |
| D�� | �ﵽƽ��ʱ����ϵ�и����ʵ�Ũ��һ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��Z��=0.45mol/L | B�� | c��X2��=0.3mol/L c��Z��=0.1 mol/L | ||
| C�� | c��X2��=0.5mol/L | D�� | c��Y2��=0.9mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ��Һ��c��Fe3+��=0.2 mol•L-1 | |
| B�� | SO42����NH4+��Na+һ�����ڣ�CO32����Al3+һ�������� | |
| C�� | ��Һ��������4�����Ӵ��ڣ�����Cl��һ�����ڣ���c��Cl������0.2 mol•L-1 | |
| D�� | ȡ����ԭ��Һ���Թ��У�����KSCN��Һ����Һ��Ѫ��ɫ��˵������Һһ��û��Fe2+��ֻ��Fe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
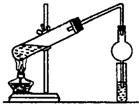 ijͬѧ���Ҵ��������Ũ������ȡ����������װ����ͼ��ʾ��
ijͬѧ���Ҵ��������Ũ������ȡ����������װ����ͼ��ʾ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com