
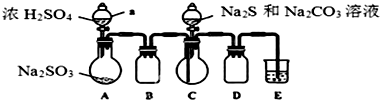
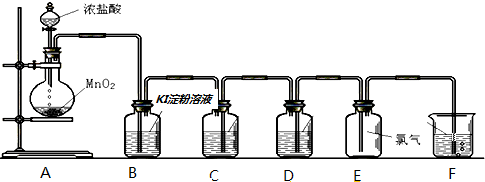
| ʵ�鲽�� | Ԥ���������� |
| ����1��ȡ����������Ʒ������������ˮ�� | ������ȫ�ܽ����ɫ������Һ |
| ����2������������� | |
| ����3�����ú�ȡ����Һ������BaCl2��Һ |
���� ʵ����ģ��Na2S2O3���ɹ��̣�Aװ�ã�Na2SO3+H2SO4��Ũ��=Na2SO4+H2O+SO2����Bװ�õ������������ã�Cװ�ã�2Na2S+Na2CO3+SO2=3Na2S2O3+CO2��Dװ���������ܾ�Ϊ�̵��ܿɷ�ֹҺ�嵹����Eװ�ã�װ����ʢ��NaOH��Һ����β����������ֹ���������ŷ���Ⱦ��������Na2CO3��Na2S��ȫ���ĺ�����Ӧ������C�л�������Һ�������ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��1������Һ�����һ��ʱ���ڲ������װ�õ������ԣ�D�������л���װ���ܷ�ֹ���������������ж�����ֱ���ſգ�Ӧ���ü�Һ���գ�
��4��Na2S2O3���壬���ܻ���Na2SO4�����ʵ��˼·��Ӧ�ȳ�ȥNa2S2O3������ϡ�����ȥ��������ƣ��پ��ú�ȡ����Һ������BaCl2��Һ����SO42-�Ĵ��ڣ�
��5����KI��KMnO4��Ӧ��KMnO4��ǿ��������KI�е�Ԫ�ر�����Ϊ�ⵥ�ʣ�KMnO4����Ԫ�ر���ԭΪMn2+��
�۲�����У����ݷ�Ӧ��2S2O32-+I2�TS4O62-+2I-�������յ�ⵥ�ʱ���ԭ������ѡ�õ�����ָʾ����Na2S2O3ˮ��ʼ��ԣ�
�ܸ��ݷ���ʽ�� MnO4-��I2��S2O32-֮��Ĺ�ϵʽ���㣮
��� �⣺��1��������װ��ɺر�A��C�з�Һ©����������E�е�������ʢˮ��ˮ���У���A��Բ����ƿ��E�е��ܿ�������ð����ֹͣ���ȣ������л���һ���ȶ���ˮ����˵����©�������������ã�D�������ܾ�Ϊ�̵��ܿɷ�ֹҺ�嵹����E��ʢ��NaOH��Һ����β����������ֹ���������ŷ��ڻ����У��Ӷ�����������
�ʴ�Ϊ���ر�A��C�з�Һ©����������E�е�������ʢˮ��ˮ���У���A��Բ����ƿ��E�е��ܿ�������ð����ֹͣ���ȣ������л���һ���ȶ���ˮ������ֹ������ ���ն����SO2��
��4�����������ȡ�����������Թ��м�ˮ�ܽ����������ᣬ��������������ᷴӦ���ɶ���������������ʣ�ȡ�ϲ���Һ���μ�����BaCl2��Һ������������ˮ�İ�ɫ�������ᱵ��֤������������ӣ���֤�������к��������ƣ�˵����Ʒ�к���SO42-��������
�ʴ�Ϊ��
| Ԥ���������� |
| ��dz��ɫ���dz��֣��д̼�����ζ������� |
| �а�ɫ����������˵����Ʒ�к���SO42- |
���� ���⿼������ʵ�鷽����ƣ����ؿ���ѧ����ʵ�鷽������ơ����ۣ���ȷ���������ǽⱾ��ؼ���ע�⣨4����Ϊ�״��㣬ͬʱ����ѧ��˼ά�������ԣ���Ŀ�Ѷ��еȣ�


 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ˮ�������c��H+��•c��OH-��=10-20����Һ�У�Na+��Cl-��S2-��SO32- | |
| B�� | �μ���ɫ��̪����Ϊ��ɫ����Һ��Na+��CO32-��K+��ClO-��SO42- | |
| C�� | ���루NH4��2Fe��SO4��2•6H2O�������Һ�У�Na+��H+��Cl-��NO3- | |
| D�� | �����£�pH��7����Һ�У�I-��SO42-��Al3+��K+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ;���ٷ�Ӧ��������Ũ�����ǿ�����Ժ����� | |
| B�� | ;���ڵĵڶ�����Ӧ��ʵ�������п���ͨ������O2Ũ������߲��� | |
| C�� | ��;���ٺ͢ڷֱ���ȡ1mol H2SO4�������ϸ�����1mol S����ת��6mol���� | |
| D�� | ;������;������ȸ������֡���ɫ��ѧ���������Ϊ;���ڱ�;������Ⱦ���С��ԭ�������ʸ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 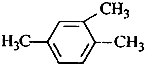 1��3��4-���ױ� 1��3��4-���ױ� | |
| B�� | 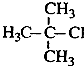 2-��-2-�ȱ��� 2-��-2-�ȱ��� | |
| C�� | 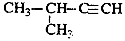 2-��-3-��Ȳ 2-��-3-��Ȳ | |
| D�� | CH3CH2C��CH3��2CH��C2H5��CH3 3��3��4-�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=0����ɫ��Һ�У�Cl-��Na+��SO42-��Fe2+ | |
| B�� | ���д���Fe3+����Һ�У�Al3+��SCN-��Br-��Na+ | |
| C�� | ���������ܷų�H2����Һ�У�Mg2+��NH4+��NO3-��Cl- | |
| D�� | ��$\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1012����Һ�У�NH4+��NO3-��K+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
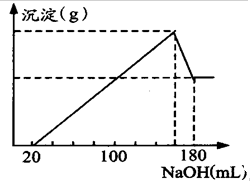 ��һ��������þ�����Ͻ�Ͷ��100mLһ�����ʵ���Ũ��HCl�У��Ͻ�ȫ���ܽ⣬��������Һ�еμ�5mol/LNaOH��Һ��������
��һ��������þ�����Ͻ�Ͷ��100mLһ�����ʵ���Ũ��HCl�У��Ͻ�ȫ���ܽ⣬��������Һ�еμ�5mol/LNaOH��Һ���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| N2 | H2 | NH3 | |
| Ͷ�Ϣ� | 1.0mol/L | 3.0mol/L | 0 |
| Ͷ�Ϣ� | 0.5mol/L | 1.5mol/L | 1.0mol/L |
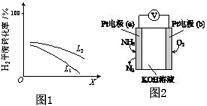
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com