
�̷�(FeSO4��7H2O )���������������ʵ�����Ͽ��Ƶ�Ħ���ξ��壬��Ӧԭ��Ϊ: (NH4)2 SO4+ FeSO4 + 6H2O =(NH4)2SO4��FeSO4��6H2O���������̿ɱ�ʾΪ:
(1)ϴ����Na2CO3����Ҫ������ ��
(2)�ᾧ������Ҫ���������ܼ���Ũ���ᾧ��Ӧ���ȵ� ʱ��ֹͣ���ȡ�
(3)����������ͼ��ʾװ�ý��еģ����ֹ��˸���ͨ������ȣ����˹����ٶȿ��⣬����һ���ŵ��� ��
(4)����ˮ�Ҵ�ϴ�ӵ�Ŀ���� ��
(5)��Ʒ��Fe2+�Ķ�������:�Ƶõ�Ħ������Ʒ���������м�������Fe3+��Ϊ�˲ⶨĦ���β�Ʒ��Fe2+�ĺ�����һ�����������������KMnO4��Һ�ζ��ķ�������ȡ4.0g��Ħ������Ʒ������ˮ������������ϡ���ᡣ��0.2 mol/LKMnO4��Һ�ζ�������Һ��Fe2+ȫ��������ʱ������KMnO4��Һ10.00mL��
�ٱ�ʵ���ָʾ���� ��(����ĸ)
A. ��̪ B.����C.ʯ��D.����Ҫ
�ڲ�Ʒ��Fe2+����������Ϊ ��
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ����һ�и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(10��)�Ի�ͭ��(��Ҫ�ɷ�ΪCuFeS2������������SiO2��)Ϊԭ�ϣ�����������ͭ��ͬʱ�õ�����Ʒ�̷�(FeSO4��7H2O)������Ҫ�������£�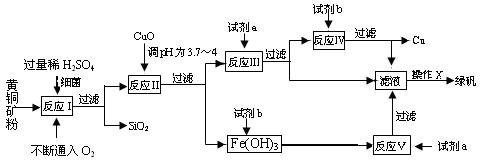

��֪����4CuFeS2��2H2SO4��17O2��4CuSO4��2Fe2(SO4)3��2H2O��
�ڲ���������������������ʽ��ʼ��������ȫ����ʱ��Һ��p H���±���
H���±���
| ������ | Cu(OH)2 | Fe(OH)3 | Fe(OH)2 |
| ��ʼ����pH | 4.7 | 2.7 | 7.6 |
| ��ȫ����pH | 6.7 | 3.7 | 9.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���㽭ʡ��һ�и����߿�ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ʵ����
(16��)��֪��������茶���Ϊdz��ɫ��������ˮ���������Ҵ�����ˮ�е��ܽ�ȱ�FeSO4��(NH4)2SO4��ҪС����ˮ�⣻���л�ԭ�ԣ��������������ȶ���
�����ǽ��̷�(FeSO4?7H2O)���������������ʵ�����Ͽ��Ƶ�Ħ���ξ��������ͼ��������ͼ�ش�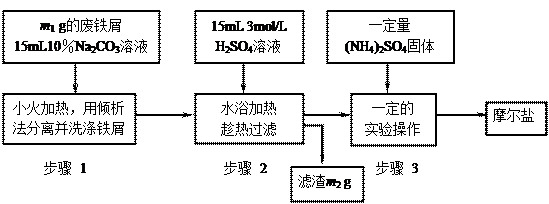
(1)Ϊ��������м��������ۣ���10��Na2CO3��Һ��ϴ���������ӷ���ʽ��ʾNa2CO3��Һ�ʼ��Ե�ԭ�� �� ��
(2)����1�в����������������м�������ʺ������������� �� ��
A.�����Ŀ����ϴ� B.�������׳��� C.�����ʽ�״ D.��������״
(3)����2 ����м������Ŀ����(�����ӷ���ʽ��ʾ)�� �� ������2����Һ���ȹ��˵�ԭ���� �� ��
����м������Ŀ����(�����ӷ���ʽ��ʾ)�� �� ������2����Һ���ȹ��˵�ԭ���� �� ��
(4)����3�У�����(NH4)2SO4�����Ӧ ���ȵ� �� ʱ��ֹͣ���ȡ��辭����ʵ����������� �� ����������������ѹ����(�����)�ȵõ���Ϊ �� �ľ��塣
���ȵ� �� ʱ��ֹͣ���ȡ��辭����ʵ����������� �� ����������������ѹ����(�����)�ȵõ���Ϊ �� �ľ��塣
(5)����װ�õ������У� �� �� �� ����ȫƿ����������ɡ�
(6)��Ʒ��Fe2���Ķ���������
�Ƶõ�Ħ������Ʒ���������м�������Fe3+��Ϊ�˲ⶨĦ���β�Ʒ��Fe2+�ĺ� ����һ���������������KMnO4��Һ�ζ��ķ�����
����һ���������������KMnO4��Һ�ζ��ķ�����
��ȡ4.0 g��Ħ������Ʒ������ˮ������������ϡ���ᡣ��0.2 mo1/L KMnO4��Һ�ζ�������Һ��Fe2+ȫ��������ʱ������KMnO4��Һ��� 10.00 mL��
������ɵζ������з��������ӷ���ʽ��
Fe2���� MnO4����( )=" " Mn2���� Fe3���� H2O
�ڱ�ʵ���ָʾ�� �� ��
A.��̪ B.���� C.ʯ�� D.����Ҫ
��KMnO4��Һ���� �� (��ʽ����ʽ)�ζ�����
���յ���ɫ�ı仯�� �� ��
�ݲ�Ʒ��Fe2+����������Ϊ �� �� (7)��25��ʱ����ͬ���ʵ���Ũ�ȵ�(NH4)2 CO3��(NH4)2SO4��Ħ������������Һa,b,c����笠�����Ũ����С���������˳��Ϊ�� �� ��(��a,b,c�ش�)
(7)��25��ʱ����ͬ���ʵ���Ũ�ȵ�(NH4)2 CO3��(NH4)2SO4��Ħ������������Һa,b,c����笠�����Ũ����С���������˳��Ϊ�� �� ��(��a,b,c�ش�)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�갲��ʡ��������У�����������������ۻ�ѧ�Ծ��������棩 ���ͣ������
ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O)���������£�
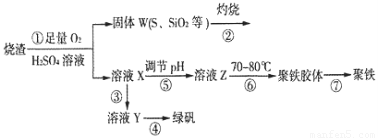
��1�������̢��еIJ���������ͨ��������Һ�У���Һ����ɫ����__________��
A��Ʒ����Һ??????? B����ɫʯ����Һ????? C������KMnO4��Һ???? D����ˮ
��2�����̢��У�FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ��___________________________________��
��3�����̢��У�������������___________________________��
��4�����̢��У������ᾧ��Ҫʹ�þƾ��ơ����Ǽܡ������ǣ�����Ҫ��������_______________��
��5�����̢ݵ���pH��ѡ�������Լ��е�___________ (��ѡ�����)��
A��ϡ����??????? B��CaCO3?????? C��NaOH��Һ
��6�����̢��У�����ҺZ���ȵ�70һ80����Ŀ����_____________________��
��7��ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣���÷�����ƽ��ȡ2.70g��Ʒ���ڽ���Ʒ�����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ3.495g�����þ�����Ҫ�ɷ�Ϊ[(Fe(OH)(SO4)]n����þ�����Ʒ����Ԫ�ص���������Ϊ___________��(���������в�����Ԫ�غ���Ԫ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ������ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��14�֣�ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O)���������£�
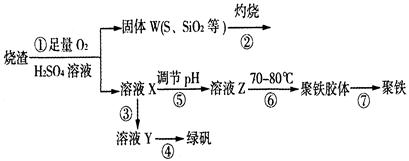
(1)�����̢��еIJ���������ͨ��������Һ�У���Һ����ɫ���� ��
A��Ʒ����Һ B����ɫʯ����Һ C������KMnO4��Һ D����ˮ
(2)���̢��У�FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ�� ��
(3)���̢��У������������� ��
(4)���̢��У������ᾧ��Ҫʹ�þƾ��ơ����Ǽܡ������ǣ�����Ҫ��������
��
(5)���̢ݵ���pH��ѡ�������Լ��е� (��ѡ�����)��
A��ϡ���� B��CaCO3 C��NaOH��Һ
(6)���̢��У�����ҺZ���ȵ�70һ80�棬Ŀ���� ��
(7)ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص�������������������ʵ�顣���÷�����ƽ��ȡ2.00g��Ʒ���ڽ���Ʒ�����������������������Ȼ�����Һ���۹��ˡ�ϴ�ӡ�����������ù�������Ϊ2.33g�����þ�����Ҫ�ɷ�Ϊ[(Fe(OH)(SO4)]n����þ�����Ʒ����Ԫ�ص���������Ϊ ��(���������в�����Ԫ�غ���Ԫ��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(10��) �Ի�ͭ��(��Ҫ�ɷ�ΪCuFeS2������������SiO2��)Ϊԭ�ϣ�����������ͭ��ͬʱ�õ�����Ʒ�̷�(FeSO4��7H2O)������Ҫ�������£�
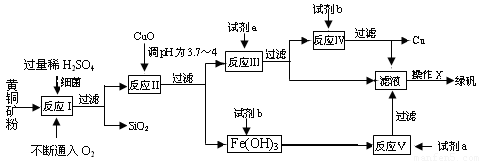
��֪����4CuFeS2��2H2SO4��17O2��4CuSO4��2Fe2(SO4)3��2H2O��
�ڲ���������������������ʽ��ʼ��������ȫ����ʱ��Һ��pH���±���
|
������ |
Cu(OH)2 |
Fe(OH)3 |
Fe(OH)2 |
|
��ʼ����pH |
4.7 |
2.7 |
7.6 |
|
��ȫ����pH |
6.7 |
3.7 |
9.6 |
(1)�Լ�a��___________���Լ�b��_____________��
(2)����XӦΪ����Ũ����________________��________________��
(3)��Ӧ���м�CuO��pHΪ3.7��4��Ŀ����
��
(4)��Ӧ�������ӷ���ʽΪ___________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com