
![]() ��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��100 g 5.00%��NaOH��Һ��������CuSO4��Һ��100 g 10.00%��K2SO4��Һ���缫��Ϊʯī�缫��
��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��100 g 5.00%��NaOH��Һ��������CuSO4��Һ��100 g 10.00%��K2SO4��Һ���缫��Ϊʯī�缫��
![]()

![]() ��1����ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӡ��ݴ˻ش����⣺
��1����ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӡ��ݴ˻ش����⣺
![]() �ٵ�Դ��N��Ϊ�ߣߣߣߣߣ���
�ٵ�Դ��N��Ϊ�ߣߣߣߣߣ���
![]() �ڵ缫b�Ϸ����ĵ缫��ӦΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
�ڵ缫b�Ϸ����ĵ缫��ӦΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
![]() ����ʽ����缫b�����ɵ������ڱ�״���µ������
����ʽ����缫b�����ɵ������ڱ�״���µ������
![]() �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
![]() �ܵ缫c�������仯�ǣߣߣߣߣߣߣߣߣ�g��
�ܵ缫c�������仯�ǣߣߣߣߣߣߣߣߣ�g��
![]() �ݵ��ǰ�����Һ���ᡢ���Դ�С�Ƿ����仯��������ԭ��
�ݵ��ǰ�����Һ���ᡢ���Դ�С�Ƿ����仯��������ԭ��
![]() ����Һ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
����Һ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
![]() ����Һ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
����Һ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
![]() ����Һ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
����Һ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
![]() ��2�������������ͭȫ����������ʱ����ܷ�������У�Ϊʲô��
��2�������������ͭȫ����������ʱ����ܷ�������У�Ϊʲô��
![]() �ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
![]()
![]()
��1�������� ��4OH��-4e��=2H2O + O2����
��ˮ���ٵ�������![]()
����O2�����![]()
��16g
�ݼ���������Ϊ����ˮ��������Һ��NaOHŨ������
����������Ϊ������OH-����O2����Һ��H+����Ũ������
����Դ�Сû�б仯����ΪK2SO4��ǿ��ǿ���Σ�Ũ�����Ӳ�Ӱ����Һ�������
(2)�ܼ������У���ΪCuSO4��Һ��ת��ΪH2SO4��Һ����ӦҲ�ͱ�Ϊˮ�ĵ�ⷴӦ��
��1��������C�缫�������ӣ���c�������ķ�ӦΪ��Cu2��+2e��=Cu����C��Ϊ�������ɴ˿��Ƴ�bΪ������aΪ������MΪ������NΪ����������ΪK2SO4���൱�ڵ��ˮ�������ˮ������Ϊxg���ɵ��ǰ��������������У�100��10%=��100-x����10.47%����x=4.5g����Ϊ0.25mol���ɷ���ʽ2H2+O2 ![]() 2H2O��֪������2molH2O��ת��4mol���ӣ�����������Ӧ��ת��0.5mol���ӣ���������·�Ǵ����ģ���ÿ���ձ��еĵ缫��ת�Ƶ���������ȵġ��ڼ���ΪNaOH���൱�ڵ��H2O������b��Ϊ������OH���ŵ磬��4OH��-4e��=2H2O + O2������ת��0.5mol���ӣ�������O2Ϊ0.5/4=0.125mol������µ����Ϊ0.125��22.4=2.8L����Cu2��+2e��=Cu��ת��0.5mol���ӣ������ɵ�m(Cu)=0.5/2 ��64 =16g���ݼ����൱�ڵ��ˮ����NaOH��Ũ������pH�����������ΪCu2���ŵ磬����ΪOH���ŵ磬����H�����࣬��pH��С������Ϊ���ˮ������K2SO4���ԣ���pH�������䡣��2��ͭȫ�����������Լ������H2SO4���е��Һ���ɵ�⡣
2H2O��֪������2molH2O��ת��4mol���ӣ�����������Ӧ��ת��0.5mol���ӣ���������·�Ǵ����ģ���ÿ���ձ��еĵ缫��ת�Ƶ���������ȵġ��ڼ���ΪNaOH���൱�ڵ��H2O������b��Ϊ������OH���ŵ磬��4OH��-4e��=2H2O + O2������ת��0.5mol���ӣ�������O2Ϊ0.5/4=0.125mol������µ����Ϊ0.125��22.4=2.8L����Cu2��+2e��=Cu��ת��0.5mol���ӣ������ɵ�m(Cu)=0.5/2 ��64 =16g���ݼ����൱�ڵ��ˮ����NaOH��Ũ������pH�����������ΪCu2���ŵ磬����ΪOH���ŵ磬����H�����࣬��pH��С������Ϊ���ˮ������K2SO4���ԣ���pH�������䡣��2��ͭȫ�����������Լ������H2SO4���е��Һ���ɵ�⡣


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�������Ƕ��и߶���ѧ������������⻯ѧ�Ծ� ���ͣ������
��14�֣���ͼ��ʾװ���У��ס��������ձ��ֱ�����ʢ��200mL����ʳ��ˮ��������AgNO3��Һ��a��b��c��d�ĸ��缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ��������d�缫����������2.16g���ݴ˻ش����⣺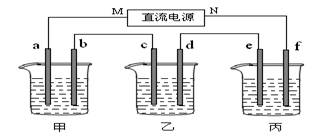
��1����Դ��N��Ϊ ����
��2���缫b�Ϸ����ĵ缫��ӦΪ ��
��3���缫c�����ɵ������ڱ�״̬�µ������ ��
��4������Һ������������Ũ��Ϊ ������Һ�����Ϊ200mL)��
��5�����ڱ��ձ���ʵ�����ı������һ��ͭ����������ҺΪ ��e�缫�IJ����ǣ� ��f�缫�ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����������ݶ��и����^��ѧ���Ի�ѧ���������� ���ͣ������
��12�֣�A��B��C����ǿ����ʣ�������ˮ�е�������������±���ʾ��
| ������ | Na+��K+��Cu2+ |
| ������ | SO42����OH�� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ��������ѧ�߶���ѧ�ڵ������¿���ѧ�Ծ����������� ���ͣ������
��10�֣���ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��100 g 5.00%��NaOH��Һ��������CuSO4��Һ��100 g 10.00%��K2SO4��Һ���缫��Ϊʯī�缫��


 ��1����ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӡ�
��1����ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47%������c�缫�������ӡ�
�ݴ˻ش����⣺ �ٵ�Դ��N��Ϊ ����
�ٵ�Դ��N��Ϊ ���� �ڵ缫b�Ϸ����ĵ缫��ӦΪ ��
�ڵ缫b�Ϸ����ĵ缫��ӦΪ �� �۵缫b�����ɵ������ڱ�״���µ������
�۵缫b�����ɵ������ڱ�״���µ������
 �ܵ缫c�������仯�� g��
�ܵ缫c�������仯�� g�� ��2�������������ͭȫ����������ʱ����ܷ�������У�Ϊʲô��
��2�������������ͭȫ����������ʱ����ܷ�������У�Ϊʲô��  ��
�� �ݵ��ǰ�����Һ��pH��α仯����������С�䣩
�ݵ��ǰ�����Һ��pH��α仯����������С�䣩 ����Һ ��
����Һ �� ����Һ�ߣߣߣߣߣߣߣ�
����Һ�ߣߣߣߣߣߣߣ� ����Һ�ߣߣߣߣߣߣߣ�
����Һ�ߣߣߣߣߣߣߣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�����и߶���ѧ������������⻯ѧ�Ծ� ���ͣ������
��14�֣���ͼ��ʾװ���У��ס��������ձ��ֱ�����ʢ��200mL����ʳ��ˮ��������AgNO3��Һ��a��b��c��d�ĸ��缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ��������d�缫����������2.16g���ݴ˻ش����⣺

��1����Դ��N��Ϊ ����
��2���缫b�Ϸ����ĵ缫��ӦΪ ��
��3���缫c�����ɵ������ڱ�״̬�µ������ ��
��4������Һ������������Ũ��Ϊ ������Һ�����Ϊ200mL)��
��5�����ڱ��ձ���ʵ�����ı������һ��ͭ����������ҺΪ ��e�缫�IJ����ǣ� ��f�缫�ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com