
1����֪X��Y��Z��WΪ���ֶ���������Ԫ�أ����ǵ�ԭ������������������X��Y��Wλ�ڲ�ͬ���ڣ�Y���γɻ�������������Ԫ�أ�Z���γ�![]() �ͻ����������W�ĵ���Ϊ��̬��
�ͻ����������W�ĵ���Ϊ��̬��
��1����X��Y��ɵ�����������Ϊijȼ�ϵ�ص� ����Ӧ�
��2��������![]() ������ѧ������Ϊ ������ ���������ӡ����ۡ�����
������ѧ������Ϊ ������ ���������ӡ����ۡ�����
��3��д��ʵ�����Ʊ�W���ʵĻ�ѧ����ʽΪ ��
�������仯����������������й㷺Ӧ�á���ش��������⣺
��1�� ������![]() �������������ұ����������Ҫԭ�ϡ�����һ����ӦΪ
�������������ұ����������Ҫԭ�ϡ�����һ����ӦΪ
![]() ,��3mol
,��3mol![]() �μӷ�Ӧ��ת�� mol���ӡ�
�μӷ�Ӧ��ת�� mol���ӡ�
��2�� �Ȼ�����Һ������ӡˢ��·ͭ�帯ʴ������Ӧ�����ӷ���ʽΪ
���Ӹ�ʴ��Һ���յõ�����ͭ������Ҫ���Լ��� ��
��3�� ���������ƣ�������Ҳ��������ˮ������ԭ����
��
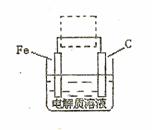
��4�������ĵ绯��ʴ��ʾ��ͼ���£�����ͼ�����ļ��ɳ�Ϊ�����绯ѧ�����ļ�ʾ��ͼ��������ͼ���߿��������ģ����ü�ͷ���������������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 NH3?H2O+H+
NH3?H2O+H+ NH3?H2O+H+
NH3?H2O+H+ H++HO2-HO2-
H++HO2-HO2- H++O22-
H++O22- H++HO2-HO2-
H++HO2-HO2- H++O22-
H++O22-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| X | Y |
| Z | W |
| A����HmXOnΪǿ�ᣬ��X���⻯������ˮһ�������ԣ�m��n��Ϊ�������� |
| B��������Ԫ�ؾ�Ϊ��������Z������������Ӧ��ˮ����һ��Ϊǿ�� |
| C��������Ԫ�ؾ�Ϊ�ǽ�������W������������Ӧ��ˮ����һ��Ϊǿ�� |
| D��������Ԫ����ֻ��һ��Ϊ��������Z��Y���ߵ�����������Ӧ��ˮ�����ܷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣�1����֪X��Y��Z��WΪ���ֶ���������Ԫ�أ����ǵ�ԭ������������������X��Y��Wλ�ڲ�ͬ���ڣ�Y���γɻ�������������Ԫ�أ�Z���γ�![]() �ͻ����������W�ĵ���Ϊ��̬��
�ͻ����������W�ĵ���Ϊ��̬��
��1����X��Y��ɵ�����������Ϊijȼ�ϵ�ص� ����Ӧ�
��2��������![]() ������ѧ������Ϊ ������ ���������ӡ����ۡ�����
������ѧ������Ϊ ������ ���������ӡ����ۡ�����
��3��д��ʵ�����Ʊ�W���ʵĻ�ѧ����ʽΪ ��
�������仯����������������й㷺Ӧ�á���ش��������⣺
��1�� ������ �������������ұ����������Ҫԭ�ϡ�����һ����ӦΪ
�������������ұ����������Ҫԭ�ϡ�����һ����ӦΪ
![]() ,��3mol
,��3mol![]() �μӷ�Ӧ��ת�� mol���ӡ�
�μӷ�Ӧ��ת�� mol���ӡ�
��2�� �Ȼ�����Һ������ӡˢ��·ͭ�帯ʴ������Ӧ�����ӷ���ʽΪ
���Ӹ�ʴ��Һ���յõ�����ͭ������Ҫ���Լ��� ��
��3�� ���������ƣ�������Ҳ��������ˮ������ԭ����
��4�������ĵ绯��ʴ��ʾ��ͼ���£�����ͼ�����ļ��ɳ�Ϊ�����绯ѧ�����ļ�ʾ��ͼ��������ͼ���߿��������ģ����ü�ͷ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��13�֣���֪X��Y��Z��WΪ���ֶ�����Ԫ�أ���ԭ��������������������ЩԪ������ɵĵ����ڳ����¾�����̬��X��Y��W���ڲ�ͬ���ڣ�����һ���������䵥���ܷ�����Ӧ���ס��ҿɻ����������ӻ���������Իش��������⣺
��1��WԪ�������ڱ��е�λ���� ���ڡ� �壻Y2�ĵ���ʽ�� ��
��2��X��Y��Zԭ�Ӱ뾶�ɴ�С˳��Ϊ ��дԪ�ط��ţ�������ˮ��Һ�����ԣ������ӷ���ʽ��ʾ��ԭ�� ��
��3��X��Z�������ԭ�ӻ����ﶡ���仯ѧʽΪ ���˷����к��еĹ��ۼ�����Ϊ ��
��4��X��Y��ɵ�Һ̬������Y2X416g�������Ķ���Ӧ����Y2��Һ̬ˮ���ų�QkJ(Q>0)����������д���÷�Ӧ���Ȼ�ѧ����ʽ ��Y2X4�����Ժ�Z2��������Ч��ȼ�ϵ�أ����������ҺΪNaOH��Һ�����ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com