
 ʵ������Ҫ0.80mol/L NaOH��Һ475mL��0.40mol/L������Һ500mL��������������Һ����������ش��������⣺
ʵ������Ҫ0.80mol/L NaOH��Һ475mL��0.40mol/L������Һ500mL��������������Һ����������ش��������⣺���� ��1����������һ�����ʵ���Ũ�ȵ���Һ�IJ���ȷ���������������жϣ�
��2������ƿ����������һ�����һ�����ʵ���Ũ�ȵ���Һ������������ʹ��ԭ�����ش�
��3����Ҫ�������Ƶ����ʵ���n=cV������Ϊm=nM������C=$\frac{n}{V}$��������������
��4��������CO2������ͨ��NaOH��Һ�����м�������NaHCO3��������������NaHCO3+NaOH=Na2CO3+H2O���ݴ˷�����
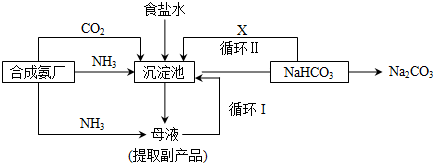
�ڸ������ƹ�����д����ѧ����ʽ��
��� �⣺��1������һ�����ʵ���Ũ�ȵ���Һ��Ҫ�������У�������ƽ����Ͳ��ҩ�ס��ձ�����������500ml������ƿ����ͷ�ιܣ�����Ҫ����Բ����ƿ����Һ©�����ʴ�Ϊ��A��C���ձ�����������
��2������ƿ����������һ�����ȷŨ�ȵı���Һ��������������ȡһ�������Һ�壬Ҳ���ܲ�������ƿ������µ����������Һ�壬����������Һ���������������ܽ�������ʣ���ѡ��BCDE��
��3����Ҫ�������Ƶ����ʵ���n=c��V=0.80mol•L-1 ��0.5L=0.4mol������Ϊm=n��M=0.4mol��40g/mol=16.0g������C=$\frac{n}{V}$��֪��������ʱ���ӿ̶��ߣ���������Һ���ƫС����Ũ��ƫ��
�ʴ�Ϊ��16.0�����ڣ�
��4��������CO2������ͨ��NaOH��Һ�����м�������NaHCO3��������������NaHCO3+NaOH=Na2CO3+H2O���ʿ���ȡ25 mL NaOH��Һͨ�������CO2����ʹ��ת��ΪNaHCO3��
���ų�������̼��Ȼ���ڵõ�����Һ�м�����һ�루25 mL��NaOH��Һ��ʹ��Һ��ֻ�ϼ��ô���̼���ƣ�
�ʴ�Ϊ��a��ȡ25 mL NaOH��Һͨ�������CO2���壬��CO2�����ܽ⣻����
b��С�������Һ1��2���ӣ��ų���Һ���ܽ��CO2���壻
c���ڵõ�����Һ�м�����һ�루25 mL��NaOH��Һ��ʹ��Һ��ֻ�ϼ��ô���̼���ƣ�
�ڸ���ʵ�鲽���֪����NaOH��Һͨ�������CO2����ʱ������Ӧ��NaOH+CO2=NaHCO3���õ�����Һ�м�����һ�루25 mL��NaOH��Һʱ��������Ӧ��NaHCO3+NaOH=Na2CO3+H2O��
�ʴ�Ϊ��NaOH+CO2=NaHCO3��NaHCO3+NaOH=Na2CO3+H2O��
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ����ȷ���õ�ԭ���Ͳ����ǽ���ؼ���ע�������������ķ����ͼ��ɣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij��ɫ����������Һ��Cl-��Na+��MnO4-��SO42- | |
| B�� | ��ʹpH��ֽ������ɫ����Һ��Na+��NH4+��K+��CO32- | |
| C�� | �������ܷų���������Һ��Na+��NO3-��Cl-��Ba2+ | |
| D�� | �μӼ����Ժ�ɫ����Һ��K+��NH4+��Cl-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | vA=0.15mol•L-1•min-1 | B�� | vB=0.6mol•L-1•min-1 | ||
| C�� | vC=0.4mol•L-1•min-1 | D�� | vD=0.01mol•L-1•s-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Ӿ����У����Ӽ��������Խ�÷���Խ�ȶ� | |
| B�� | ���Ӿ����У����ۼ��ļ���Խ���ۡ��е�Խ�� | |
| C�� | ԭ�Ӿ����У����ۼ��ļ���Խ���ۡ��е�Խ�� | |
| D�� | ij��������ˮ�ɵ���������ƶ������ӣ��þ���һ�������Ӿ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��Na+[��H]-��дһ����
��Na+[��H]-��дһ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
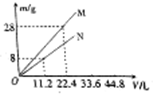
| A�� | 1��1 | B�� | 2��7 | C�� | 7��4 | D�� | 7��2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com