
ÓŠĻĀĮŠ»ÆѧŅĒĘ÷£ŗ
¢ŁĶŠÅĢĢģĘ½””¢Ś²£Į§°ō””¢ŪŅ©³×””¢ÜÉÕ±””¢ŻĮæĶ²””¢ŽČŻĮæĘæ””¢ß½ŗĶ·µĪ¹Ü””¢ąĻøæŚŹŌ¼ĮĘæ””¢į±źĒ©Ö½
(1)ĻÖŠčŅŖÅäÖĘ500 mL 1 mol”¤L£1 H2SO4ČÜŅŗ£¬ŠčŅŖÖŹĮæ·ÖŹżĪŖ98%”¢ĆܶČĪŖ1.84 g”¤cm£3µÄÅØH2SO4________ mL”£
(2)ÉĻŹöŅĒĘ÷ÖŠ£¬°“ŹµŃéŅŖĒóŹ¹ÓƵÄĻČŗóĖ³Šņ£¬Ę䱹ŗÅÅÅĮŠŹĒ____________________”£
(3)ČŻĮæĘæŹ¹ÓĆĒ°¼ģŃéŹĒ·ńĀ©Ė®µÄ·½·ØŹĒ____________________________
__________________________________________________________ӣ
(4)ČōŹµŃé¹ż³ĢÖŠÓöµ½ĻĀĮŠĒéæö£¬¶ŌĮņĖįµÄĪļÖŹµÄĮæÅضČÓŠŗĪÓ°Ļģ(Ģī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°²»±ä”±)”£
¢ŁÓĆĄ“Ļ”ŹĶÅØĮņĖįµÄÉÕ±Ī“Ļ“µÓ£¬________£»
¢ŚĪ“¾ĄäČ“½«ČÜŅŗ×¢ČėČŻĮæĘæÖŠ£¬________£»
¢ŪŅ”ŌČŗó·¢ĻÖŅŗĆęĻĀ½µŌŁ¼ÓĖ®£¬________£»
¢Ü¶ØČŻŹ±ø©ŹÓ¹Ū²ģŅŗĆę£¬________”£
½āĪö ””±¾ĢāÖ÷ŅŖæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ¼°Īó²ī·ÖĪö”£±¾ĢāÄѵćŌŚÓŚĪó²ī·ÖĪö£¬Ąķ½āŅżĘšĪó²īµÄŌŅņŹĒ½āĢāµÄ¹Ų¼ü”£øł¾ŻĻ”ŹĶ¹ż³ĢÖŠČÜÖŹµÄĪļÖŹµÄĮæ²»±äæÉĒóĖćĖłŠčÅØH2SO4µÄĮ棻ÉÕ±Ī“Ļ“µÓ£¬ÉÕ±ÉĻ½«Õ“ÓŠĮņĖį£»ČÜŅŗĪ“ĄäČ“£¬ČÜŅŗĢå»żÅņÕĶ£¬ĄäČ“ŗóČÜŅŗƻӊµ½“ļæĢ¶ČĻߣ»Ņ”ŌČŗó·¢
””±¾ĢāÖ÷ŅŖæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ¼°Īó²ī·ÖĪö”£±¾ĢāÄѵćŌŚÓŚĪó²ī·ÖĪö£¬Ąķ½āŅżĘšĪó²īµÄŌŅņŹĒ½āĢāµÄ¹Ų¼ü”£øł¾ŻĻ”ŹĶ¹ż³ĢÖŠČÜÖŹµÄĪļÖŹµÄĮæ²»±äæÉĒóĖćĖłŠčÅØH2SO4µÄĮ棻ÉÕ±Ī“Ļ“µÓ£¬ÉÕ±ÉĻ½«Õ“ÓŠĮņĖį£»ČÜŅŗĪ“ĄäČ“£¬ČÜŅŗĢå»żÅņÕĶ£¬ĄäČ“ŗóČÜŅŗƻӊµ½“ļæĢ¶ČĻߣ»Ņ”ŌČŗó·¢ ĻÖŅŗĆęĻĀ½µ²»ÄÜŌŁ¼ÓĖ®£»¶ØČŻŹ±£¬ø©ŹÓ¹Ū²ģŅŗĆę£¬Ć»ÓŠµ½“ļæĢ¶ČĻߣ¬Ģå»żĘ«Š””£
ĻÖŅŗĆęĻĀ½µ²»ÄÜŌŁ¼ÓĖ®£»¶ØČŻŹ±£¬ø©ŹÓ¹Ū²ģŅŗĆę£¬Ć»ÓŠµ½“ļæĢ¶ČĻߣ¬Ģå»żĘ«Š””£
“š°ø””(1)27.2””(2)¢Ż¢Ü¢Ś¢Ž¢ß¢ą¢į””(3)ĶłČŻĮæĘæÖŠ¼ÓŹŹĮæĖ®£¬ČūŗĆĘæČū£¬ÓĆŹ³Öø¶„×”ĘæČū£¬ÓĆĮķŅ»Ö»ŹÖµÄĪåÖøĶŠ×”Ęæµ×£¬°ŃĘæµ¹Į¢¹żĄ“£¬Čē²»Ā©Ė®£¬°ŃĘæČūŠż×Ŗ180”ćŗóČū½ō£¬ŌŁ°ŃĘæµ¹Į¢¹żĄ“£¬Čō²»Ā©Ė®£¬²ÅÄÜŹ¹ÓĆ””(4)¢ŁĘ«µĶ””¢ŚĘ«øß””¢ŪĘ«µĶ””¢ÜĘ«øß


 ѧĮ·æģ³µµĄæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
ѧĮ·æģ³µµĄæŚĖćŠÄĖćĖŁĖćĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
³ōŃõ²ćŹĒµŲĒņÉśĆüµÄ±£»¤Éń£¬³ōŃõ±ČŃõĘų¾ßÓŠøüĒæµÄŃõ»ÆŠŌ”£ŹµŃéŹŅæɽ«ŃõĘųĶعżøßŃ¹·Åµē¹ÜĄ“ÖĘČ”³ōŃõ£ŗ3O2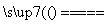 2O3”£
2O3ӣ
(1)ČōŌŚÉĻŹö·“Ó¦ÖŠÓŠ30%µÄŃõĘų×Ŗ»ÆĪŖ³ōŃõ£¬ĖłµĆ»ģŗĻĘųµÄĘ½¾łÄ¦¶ūÖŹĮæĪŖ________ g”¤mol£1(±£ĮōŅ»Ī»Š”Źż)”£
(2) ½«8 LŃõĘųĶعż·Åµē¹Üŗ󣬻Öø“µ½Ōדæö£¬µĆµ½ĘųĢå6.5 L£¬ĘäÖŠ³ōŃõĪŖ________ L”£
½«8 LŃõĘųĶعż·Åµē¹Üŗ󣬻Öø“µ½Ōדæö£¬µĆµ½ĘųĢå6.5 L£¬ĘäÖŠ³ōŃõĪŖ________ L”£
(3)ŹµŃéŹŅ½«ŃõĘųŗĶ³ōŃõµÄ»ģŗĻĘųĢå0.896 L(±ź×¼×“æö)ĶØČėŹ¢ÓŠ20.0 gĶ·ŪµÄ·“Ó¦Ę÷ÖŠ£¬³ä·Ö¼ÓČČŗ󣬷ŪÄ©µÄÖŹĮæ±äĪŖ21.6 g”£ŌņŌ»ģŗĻĘųÖŠ³ōŃõµÄĢå»ż·ÖŹżĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)½šøÕĶéµÄ½į¹¹ČēĶ¼ĖłŹ¾£¬ĖüæÉæ“×÷ŹĒÓÉĖÄøöµČĶ¬µÄĮłŌŖ»·×é³ÉµÄæռ乹ŠĶ”£

½šøÕĶé
¢Ł½šøÕĶéµÄ·Ö×ÓŹ½ĪŖ________£»
¢Śøł¾ŻÖŠŃ§Ń§¹żµÄĶ¬·ÖŅģ¹¹ĢåÅŠ¶Ļ¹ęŌņ£¬ÅŠ¶ĻÓÉäåŌ×ÓČ”“ś·Ö×ÓÖŠµÄĒāŌ×ÓŠĪ³ÉµÄŅ»äå“śĪļÓŠ________ÖÖ”£
(2)”°Į¢·½Ķé”±ŹĒŅ»ÖÖŠĀŗĻ³ÉµÄĢž£¬Ęä·Ö×ÓĪŖÕż·½Ģå½į¹¹£¬ĘäĢ¼¼Ü½į¹¹ČēĶ¼ĖłŹ¾”£
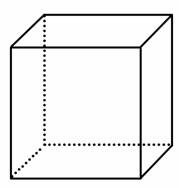
¢ŁĮ¢·½ĶéµÄ·Ö×ÓŹ½ĪŖ________£»
¢ŚøĆĮ¢·½ĶéµÄ¶žĀČ“śĪļ¾ßÓŠĶ¬·ÖŅģ¹¹ĢåµÄŹżÄæŹĒ______£»
¢ŪøĆĮ¢·½ĶéµÄĮłĀČ“śĪļ¾ßÓŠĶ¬·ÖŅģ¹¹ĢåµÄŹżÄæŹĒ______£»
¢Ü½«a gĮ¢·½ĶéČÜÓŚb mL±½ÖŠ£¬Č»ŗóĶØČėc LŅŅČ²(±ź×¼×“æöĻĀ)£¬ĖłµĆ»ģŗĻĪļÖŠĢ¼µÄ°Ł·Öŗ¬ĮæĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠČÜŅŗÖŠ£¬ČÜÖŹµÄĪļÖŹµÄĮæÅضČĪŖ1 mol”¤L£1µÄŹĒ
A£®½«40 g NaOHČÜÓŚ1 LĖ®ĖłµĆµÄČÜŅŗ
B£®½«80 g SO3ČÜÓŚĖ®²¢Åä³É1 LµÄČÜŅŗ
C£®½«0.5 mol”¤L£1µÄNaNO3ČÜŅŗ100 mL¼ÓČČÕō·¢µō50 gĖ®µÄČÜŅŗ
D£®ŗ¬K£«ĪŖ2 molµÄK2S O4ČÜŅŗ
O4ČÜŅŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijµŲĖįÓź¾¼ģŃé³żŗ¬H£«Ķā[c(OH£)æÉŗöŹÓ]»¹ÓŠNa£«”¢ Cl£”¢NH ”¢SO
”¢SO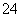 £¬ĘäÅضČŅĄ“ĪĪŖ£ŗc(Na£«)£½2.3”Į10£5 mol”¤L£1£¬c(Cl£)£½3.5”Į10£5 mol”¤L£1£¬c(NH
£¬ĘäÅضČŅĄ“ĪĪŖ£ŗc(Na£«)£½2.3”Į10£5 mol”¤L£1£¬c(Cl£)£½3.5”Į10£5 mol”¤L£1£¬c(NH )£½2.3”Į10£5 mol”¤L£1£¬c(SO
)£½2.3”Į10£5 mol”¤L£1£¬c(SO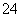 )£½1.05”Į10£5 mol”¤L£1£¬ŌņøƵŲĖįÓźµÄpHĪŖ
)£½1.05”Į10£5 mol”¤L£1£¬ŌņøƵŲĖįÓźµÄpHĪŖ
A£®3 B£®4
C£®5 D£®6
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠĮņĖįĆ¾ČÜŅŗ500 mL£¬ĖüµÄĆܶȏĒ1.20 g”¤cm£3£¬ĘäÖŠĆ¾Ąė×ÓµÄÖŹĮæ·ÖŹżŹĒ4.8%£¬ŌņÓŠ¹ŲøĆČÜŅŗµÄĖµ·Ø²»ÕżČ·µÄŹĒ
A£®ČÜÖŹµÄÖŹĮæ·ÖŹżŹĒ24.0%
B£®ČÜŅŗµÄĪļÖŹµÄĮæÅØ¶ČŹĒ2.4 mol”¤L£1
C£®ČÜÖŹŗĶČܼĮµÄĪļÖŹµÄĮæÖ®±ČŹĒ1”Ć40
D£®ĮņĖįøłĄė×ÓµÄÖŹĮæ·ÖŹżŹĒ19.2%
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ņ»¶ØĢõ¼žĻĀ£¬ŌŚŗćČŻĆܱÕČŻĘ÷ÖŠ£¬ÄܱķŹ¾·“Ó¦X(g)£«2Y(g)  2Z(g)Ņ»¶Ø“ļµ½»ÆŃ§Ę½ŗāדĢ¬µÄŹĒ(””””)
2Z(g)Ņ»¶Ø“ļµ½»ÆŃ§Ę½ŗāדĢ¬µÄŹĒ(””””)
¢ŁX”¢Y”¢ZµÄĪļÖŹµÄĮæÖ®±ČĪŖ1”Ć2”Ć2
¢ŚX”¢Y”¢ZµÄÅØ¶Č²»ŌŁ·¢Éś±ä»Æ
¢ŪČŻĘ÷ÖŠµÄŃ¹Ēæ²»ŌŁ·¢Éś±ä»Æ
¢Üµ„Ī»Ź±¼äÄŚÉś³Én mol Z£¬Ķ¬Ź±Éś³É2n mol Y
A£®¢Ł¢Ś””””””””””””””””””””B£®¢Ł¢Ü
C£®¢Ś¢Ū D£®¢Ū¢Ü
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com