


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��̼���� | B���������� |
| C��̼������ | D���Ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������NO3-��ˮ��Һ�У�NH4+��Fe2+��SO42-��H+ |
| B������CO32-�ij�������Һ�У�K+��NO3-��Cl-��Na+ |
| C����pH=2����Һ�У�ClO-��SO32-��Na+��K+ |
| D���������۷�Ӧ������������Һ�У�Na+��Al3+��CH3COO-��I- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
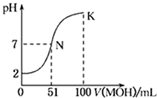 �����£���l00mL 0.01mol/L HA����Һ����μ���0.02mol/L MOH��Һ����ͼ��ʾ�����߱�ʾ�����Һ��pH�仯�������Һ����仯���Բ��ƣ�������˵������ȷ���ǣ�������
�����£���l00mL 0.01mol/L HA����Һ����μ���0.02mol/L MOH��Һ����ͼ��ʾ�����߱�ʾ�����Һ��pH�仯�������Һ����仯���Բ��ƣ�������˵������ȷ���ǣ�������| A��HAΪһԪǿ�ᣬMOHΪһԪǿ�� |
| B��N��ˮ�ĵ���̶�С��K��ˮ�ĵ���̶� |
| C��K����ʾ��Һ��c��A-����c��M+�� |
| D��K���Ӧ����Һ�У���c��MOH��+c��M+��=0.01mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ũ�������ڴ�����������ɫϸ��ƿ�� |
| B�������ⴢ���ھƾ��� |
| C���������ƹ��屣���ڴ������Ĺ��ƿ�� |
| D�����������Ʊ�����ú�͡����Ȼ�̼���л��ܼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ڢۢ� | B���ڢ� |
| C���٢ۢ� | D���ۢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ʒ�� | �߲��մ�� |
| ���� | ��ۡ��ʼ���������ʳ��ֲ���͡���ɰ�ǡ����͡�ʳ�Ρ���ˮ��ˡ���֭ |
| ������ | ʮ������ |
| �������� | 2008��11��6�� |
| A�����������ʵ����ʼ��� |
| B������ά���ص�����ˮ��˺ͳ�֭ |
| C���������۵�����ۺͰ�ɰ�� |
| D��������֬���Ǿ���ʳ��ֲ���ͺ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | C | N | O | F | ||||
| 3 | Na | Mg | Al | Si | S | Cl | Ar |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com