
��7�֣���֪�����Ȼ�ѧ����ʽ��
��H2(g)+1/2O2(g)=H2O(l) ��H= ��285 kJ/mol
��H2(g)+1/2O2(g)=H2O(g) ��H= ��241.8 kJ/mol
��C(s)+1/2O2(g)=CO(g) ��H= ��241.8 kJ/mol
��C(s)+O2(g)=CO2(g) ��H= ��393.5 kJ/mol
�ش��������⣺
��1��������Ӧ�����ڷ��ȷ�Ӧ����_________________��
��2��H2��ȼ����Ϊ___________��C��ȼ����Ϊ___________��
��3��ȼ��10 g H2����Һ̬ˮ���ų�������Ϊ__________________��
��4��CO��ȼ����Ϊ______________�����Ȼ�ѧ����ʽΪ______________________��
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
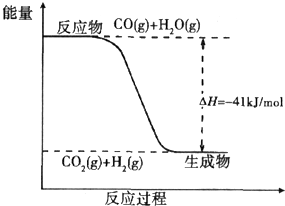 ú̿����ת��Ϊ�����Դ�ͻ���ԭ�ϣ�
ú̿����ת��Ϊ�����Դ�ͻ���ԭ�ϣ�| 1 | 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com