
| 112 |
| 160 |
| 112 |
| 160 |
| ||
| ||
| 112 |
| 160 |
| 112 |
| 160 |
a-
| ||
| a |
| a-0.7b |
| a |
| 100a-70b |
| a |
| 100a-70b |
| a |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��D��E��F��G��ԭ���������������ǰ�����ڵ�Ԫ�أ�����A���γɻ���������Ԫ��֮һ�������䳣�����ϼ�Ϊ+1��-1��BԪ�ػ�̬ԭ�Ӽ۵����Ų�ʽΪnsnnpn��D�ǵؿ��к�������Ԫ�أ�E����������������ԣ�F3+��3d�ܼ�Ϊ�������GD�Ǻ�ɫ���壬G2DΪש��ɫ���壬�ش��������⣺
A��B��C��D��E��F��G��ԭ���������������ǰ�����ڵ�Ԫ�أ�����A���γɻ���������Ԫ��֮һ�������䳣�����ϼ�Ϊ+1��-1��BԪ�ػ�̬ԭ�Ӽ۵����Ų�ʽΪnsnnpn��D�ǵؿ��к�������Ԫ�أ�E����������������ԣ�F3+��3d�ܼ�Ϊ�������GD�Ǻ�ɫ���壬G2DΪש��ɫ���壬�ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ҹ��ڷ��˷ܼ������ϵ������Ǽ��֧�֡����İ��ˡ��ģ�ij���˷ܼ��Ľṹ��ͼ��ʾ����������������ȷ���ǣ�������
�ҹ��ڷ��˷ܼ������ϵ������Ǽ��֧�֡����İ��ˡ��ģ�ij���˷ܼ��Ľṹ��ͼ��ʾ����������������ȷ���ǣ�������| A�����ķ���ʽΪC20H22O3 |
| B����������ˮ |
| C�����ķ����й�ƽ���̼ԭ�������16�� |
| D��1mol��������7molH2�ͺ�4mol Br2����ˮ������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
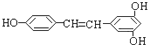 ���㷺������ʳ���ɣ�ء����������ѣ��У������ܾ��п����ԣ��ܹ���1mol�û�������Ӧ��Br2��H2����������ֱ��ǣ�������
���㷺������ʳ���ɣ�ء����������ѣ��У������ܾ��п����ԣ��ܹ���1mol�û�������Ӧ��Br2��H2����������ֱ��ǣ�������| A��1mol��1mol |
| B��3.5mol��7mol |
| C��5mol��7mol |
| D��6mol��7mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
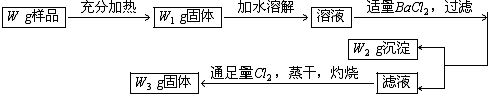
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
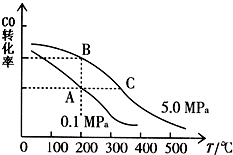 ��1����֪��C��s��+O2��g��=CO2��g����H1=-393.5kJ/mol
��1����֪��C��s��+O2��g��=CO2��g����H1=-393.5kJ/mol�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������ƽ��ȡ11.7 g CuO��ĩ |
| B���ù㷺��pH��ֽ�����Һ��pHΪ6.2 |
| C���¶ȼ���ʾ�����¶���Ϊ25.69�� |
| D����100 mL��Ͳ��ȡ5.6 mL��ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
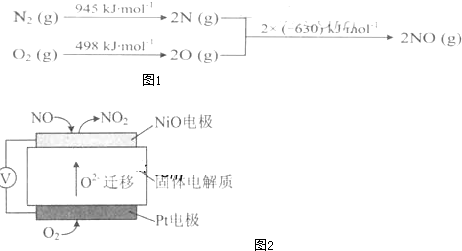
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com