
ij�����ĵ�������к���ͭ�����Ƚ��������Ϊʵ����Դ�Ļ������ò���Ч��ֹ������Ⱦ��������¹������̣�
��1����������H2O2��Ŀ���� ����pH�����м�����Լ������ ���ѧʽ����ʵ���ҽ��й��˲������õ��IJ��������� ��
��2�����CuSO4��Һ��ԭ���� ����CuSO4��Һ�м���һ������NaCl��Na2SO3���������ɰ�ɫ��CuCl������д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3����ȡ���Ʊ���CuCl��Ʒ0.2500g����һ������0.5mol��L-1FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20mL����0.1000mol��L-1��Ce��SO4��2��Һ�ζ��������յ�ʱ����Ce��SO4��2��Һ25.00mL���йصĻ�ѧ��ӦΪ��Fe3����CuCl��Fe2����Cu2����Cl����Ce4����Fe2����Fe3����Ce3���������CuCl��Ʒ���������� ��
��4��Fe3+����ˮ�ⷴӦFe3����3H2O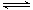 Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ������ʽΪ ��
Fe(OH)3��3H�����÷�Ӧ��ƽ�ⳣ������ʽΪ ��
��1����Fe2+������Fe3�������ڵ���pHֵ��Cu2+���룻CuO��Cu(OH)2�ȣ�©�����ձ���������
��2��������Һ�е�H2O2������Ӱ����һ��CuCl������
2CuSO4��2NaCl��Na2SO3��H2O��2CuCl����2Na2SO4��H2SO4
��3��99.50% ��4��K��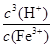
������������������ڵ�������к���ͭ�����Ƚ�����������Լ���ϡ��������Fe���������������Ӧ�������ܽ���ˮ�����ʣ���ȥ�����Ե����ʡ��ټ���H2O2������Fe2+����ΪFe3+.���ڵ�����Һ��pHֵ��ֽ��Cu2+���з��롣���ܽ�����Һ�����ԣ��ֲ������µ����ʣ�Ӧ�ü�������ʽ�������������Һ�е���ͬ���ɡ������CuO��Cu(OH)2��CuCO3��.��ʵ���ҽ��й��˲������õ��IJ���������©�����ձ�������������2������ϡ�����ܽ�����Һ�м�������ǿ�����Ե�H2O2���������ȥ����ʹ��ȡ��CuCl���������ʡ�����Ҫ��CuSO4��Һ���������H2O2����CuSO4��Һ�м���һ������NaCl��Na2SO3�����ɰ�ɫ��CuCl�����Ļ�ѧ����ʽΪ2CuSO4��2NaCl��Na2SO3��H2O��2CuCl����2Na2SO4��H2SO4����3���ɷ���ʽ�ɵù�ϵʽΪCuCl����Fe2������Ce4����n(Ce4��)="n(CuCl)=" c��V="0.1000mol/L" ��0.025L= 2.5��10-3mol.����m=n��M=2.5��10-3mol��99. 5=0.24875g.���Ը�CuCl��Ʒ����������Ϊ����0.24875�� 0.2500g����100%=99.50%����4����ѧƽ�ⳣ���ǿ��淴Ӧ�ﵽƽ��״̬ʱ��������Ũ����ָ���ij˻������Ӧ��Ũ�ȵ���ָ���˻��ıȡ���˸�ˮ�ⷴӦ��ƽ�ⳣ��K��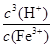 ��
��
���㣺���黯ѧʵ�������������ѧƽ�ⳣ���ı���ʽ�����ʵĴ��ȵļ��㼰��ҵ���̵�֪ʶ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������������������������Ҫ��Ӧ�ã�������ʹ�ø���IJ��Ǵ����������ǺϽ�
��1�������ӷ�Ӧ�ѵ��Ƚ������õ����ƼغϽ��䳣������ ̬��������Һ���̣������Ƶ����ʵ�������Ϊ20%��0.2mol�Ĵ˺Ͻ�ȫ�ؼ��뵽��ˮ��D2O���У���������������������������Ϊ ��
��2��þ���Ͻ��Ǿ������������ʺϽ𡣼�һ��Ͻ��ڿ�����ȼ�գ�������MgO��Al2O3�⣬���п������ɵĵ��������ʵĵ���ʽ�� ����һ��5.1g��þ���Ͻ�Ƭ����3.6 mol��L-1��200ml ��������Һ�У����������1 mol��L-1������������Һ����� mL�������������ٸı䣬��������������0.5mol �ĵ��ӷ���ת�ƣ���Ͻ���Mg�����ʵ�������Ϊ ��
��3������һ��ͭ�ĺϽ�ͭ���ɿ�����Cu��Zn�����ɷֱ������ܷ�����ܷ�����������ֽ��������аѸúϽ�Ͷ�뵽ϡ�����У����ֲ������ݵ��ٶȱ���п�����ᷴӦ���������ٶȿ죬��ԭ���� ��
��Ϊ����ijͭ�Ͻ�ijɷ֣����Ὣ����ȫ�ܽ����NaOH��Һ��pH����pH��3.4ʱ��ʼ���ֳ������ֱ���pHΪ7.0��8.0ʱ���˳����������ͼ��Ϣ�ƶϸúϽ��г�ͭ��һ������ ��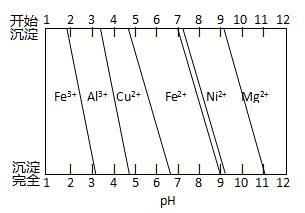
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����ij����(����Cu2O��Al2O3��Fe2O3��SiO2)��ȡͭ�IJ����������£�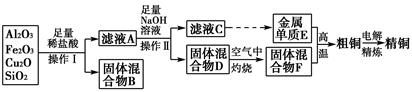
��֪��Cu2O��2H��=Cu��Cu2����H2O
(1)ʵ������������Ϊ________���ڿ��������չ�������Dʱ���õ����ֹ������ʵ������������������ƾ��ơ��������⣬����________(����������)��
(2)��ҺA����Ԫ�صĴ�����ʽΪ________(�����ӷ���)�����ɸ����ӵ����ӷ���ʽΪ____________________________________________��������ҺA�д��ڸ����ӵ��Լ�Ϊ________(���Լ�����)��
(3)��������E���������F������ijһ��Ӧ�����ں��Ӹֹ죬�÷�Ӧ�Ļ�ѧ����ʽΪ__________________________________________________��
(4)�����£���pH��NaAlO2��NaOH������Һ�У���ˮ�������c(OH��)ǰ��Ϊ���ߵ�108������������Һ��pH��________��
(5)��Ũ���ᡢŨ���ᡢ����ˮ��ѡ�ú��ʵ��Լ����ⶨ��ͭ��Ʒ�н���ͭ�������������漰����Ҫ���裺��ȡһ����������Ʒ��________________�����ˡ�ϴ�ӡ����������ʣ�����ͭ��������(��ȱ�ٵIJ������裬���������������̵�ϸ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CuSO4��5H2O��ͭ����Ҫ��������Ź㷺��Ӧ�á�������CuSO4��5H2O��ʵ�����Ʊ�����ͼ��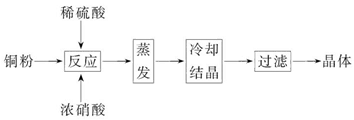
�����������������գ�
(1)��ͭ�۵�ϡ�����еμ�Ũ���ᣬ��ͭ���ܽ�ʱ���Թ۲쵽��ʵ������_____________��_____________��
(2)���ͭ�ۡ����ἰ���ᶼ�Ƚϴ��������Ƶõ�CuSO4��5H2O�п��ܴ��ڵ�������_____________����ȥ�������ʵ�ʵ�������Ϊ_____________��
(3)��֪��CuSO4+2NaOH=Cu(OH)2��+Na2SO4����ȡ0.100 0 g�ᴿ���CuSO4��5H2O��������ƿ�У�����0.100 0 mol/L����������Һ28.00 mL����Ӧ��ȫ����������������
0.100 0 mol/L����ζ����յ㣬��������20.16 mL����0.100 0 g�������к�CuSO4��5H2O_____________g��
(4)�����ζ��У��ζ�����ע������֮ǰ����������ˮϴ��������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F ��������������ͼ��ʾת����ϵ����������D������Ϊ���壬����E�ǵ���ɫ��ĩ�������ַ�Ӧ������P��Ӧ��������ȥ��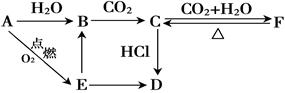
��1��д������A��D�Ļ�ѧʽ��A�� �� D�� ��
��2��д����������ת����Ӧ�����ӷ���ʽ��
B C�� ��
C�� ��
E B�� ��
B�� ��
C F�� ��
F�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�����A������Al2(SO4)3��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ�ı仯��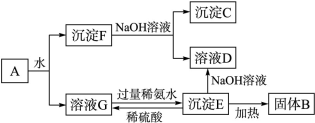
��ش��������⡣
��1�� ͼ���漰������Һ������ķ�����____________________________��
��2�� B��C��D��E�������ʵĻ�ѧʽΪ��
B__________��C__________��D__________��E__________��
��3�� д�����з�Ӧ�����ӷ���ʽ��
����F��NaOH��Һ��Ӧ________________________________��
����E��ϡ���ᷴӦ____________________________��
��ҺG�����ϡ��ˮ��Ӧ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧ��FeCl3���������Լ�FeSO4�����ȶ��Խ�������̽����
��1����������FeSO4���·ֽ⣬��Ӧ����ʽΪ�� 2FeSO4 Fe2O3+SO2��+SO3����
Fe2O3+SO2��+SO3����
����˫���ű�����·ֽ�FeSO4�Ʊ�Fe2O3��Ӧ�е���ת�Ƶķ������Ŀ ��
��Ϊ�ռ�SO3����֤SO2��Ӧ����������ͨ����û�� �е�U�ܡ�ϴ��ƿ�е� ��NaOH��Һ��
��2��ʵ��̽��Fe3+�������ԣ���FeCl3��Һ��ͨ��һ������SO2���壬��Һ�ɻ�ɫ��Ϊdz��ɫ��
��dz��ɫ��Һ��һ�����ڵ�������H+��Cl-�� �����ܴ��ڵ����� (��д���)��
A��Fe3+ B��Fe2+ C��SO42- D��H2SO3
��Ϊȷ�Ͽ��ܴ��ڵ�����Ӧѡ����Լ��� (��д���)��
A��ϡ���� B��NaOH��Һ C��KSCN��Һ D��Ʒ����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�п�������ǻ��ý����������������������ǿ�ᣬ��������ǿ������������������ڰ�ˮ��������п���ڰ�ˮ����Zn��NH3��22�����ش��������⣺
��1����������������������Һ����Һ����Ԫ�صĴ�����ʽΪ ���û�ѧʽ����� ��2��д��п������������Һ��Ӧ�Ļ�ѧ����ʽ ��
��3�����и����е�������Һ������μӵ�ʵ�鷽�����ɼ������ ��
������������������ ���������Ͱ�ˮ ������п���������� ������п�Ͱ�ˮ
��4��д�������������백ˮ��Ӧ�����ӷ���ʽ____________________________________________�� �Խ�����ʵ���Ҳ������ÿ�����п���백ˮ��Ӧ�Ʊ�������п��ԭ��___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������A��B�ͷǽ�������C��D�Լ����ǻ�����֮���ת����ϵ���¡�F��J��������ǿ��M��������ǿ��N��Z��Ħ������Ϊ198 g��mol��1�������и�Ԫ�ص�������Ϊ����:����B:����39:28:32��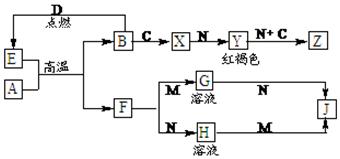
��ش��������⣺
��1��C�Ļ�ѧʽΪ ��Z�Ļ�ѧʽΪ ��
��2��д������X�������ӵķ��� ��
��3��д��E��A�ڸ����·�Ӧ�Ļ�ѧ����ʽΪ ��
��4��д��A��N��Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com