

| A�� | HA��HB��HD������ | |
| B�� | P��ʱ��c��B-����c��Na+����c��HB����c��H+����c��OH-�� | |
| C�� | pH=7ʱ��������Һ�У�c��A-��=c��B-��=c��D-�� | |
| D�� | ��������Һ�ֱ�NaOH��Һǡ����ȫ�кͺ죺c��HA��+c��HB��+c��HD���Tc��OH-��-c��H+�� |
���� A�������£�0.1mol/L��һԪ�����pH=1��˵��������ȫ���룬Ϊǿ�ᣬ���pH��1��˵�����Ჿ�ֵ��룬Ϊ���
B������ͼ֪��������100mLNaOH��Һʱ�����ǡ����ȫ��Ӧ�������50mLNaOH��Һʱ����һ�����μӷ�Ӧ����P����Һ������Ϊ�����ʵ���Ũ�ȵ�c��HB����c��NaB���������Һ�����ԣ�˵��HB����̶ȴ���NaBˮ��̶ȵ�����Һ��c��H+����c��OH-�������ݵ���غ�֪��c��B-����c��Na+�������Ჿ�ֵ��룻
C��������pH=7ʱ��Һ�����ԣ���c��H+��=c��OH-�������ݵ���غ��c��Na+���ֱ���ڸ��������Ũ�ȣ�����������Һ���������Խǿ�����ĵļ����Խ��
D���κε������Һ�ж����������غ㣬���������غ��жϣ�
��� �⣺A�������£�0.1mol/L��һԪ�����pH=1��˵��������ȫ���룬Ϊǿ�ᣬ���pH��1��˵�����Ჿ�ֵ��룬Ϊ���ᣬ����ͼ֪��δ�������Һʱ���������pH������1��˵�������ֵ��룬�������ᣬ��A��ȷ��
B������ͼ֪��������100mLNaOH��Һʱ�����ǡ����ȫ��Ӧ�������50mLNaOH��Һʱ����һ�����μӷ�Ӧ����P����Һ������Ϊ�����ʵ���Ũ�ȵ�c��HB����c��NaB���������Һ�����ԣ�˵��HB����̶ȴ���NaBˮ��̶ȵ�����Һ��c��H+����c��OH-�������ݵ���غ�֪��c��B-����c��Na+�������Ჿ�ֵ��룬��������Ũ�ȴ�С˳����c��B-����c��Na+����c��HB����c��H+����c��OH-������B��ȷ��
C��������pH=7ʱ��Һ�����ԣ���c��H+��=c��OH-�������ݵ���غ��c��Na+���ֱ���ڸ��������Ũ�ȣ�����������Һ���������Խǿ�����ĵļ����Խ������ǿ��˳����HA��HB��HD�����Ը���ﵽ����ʱ��Ҫ�����HA��HB��HD�������������Ũ�ȴ�С˳����c��A-����c��B-����c��D-������C����
D���κε������Һ�ж����������غ㣬������Һ�������غ�Ϊc��HA��+c��H+��=c��OH-����c��HB��+c��H+��=c��OH-����c��HC��+c��H+��=c��OH-������������Һ��Ϻ��c��HA��+c��HB��+c��HD���Tc��OH-��-c��H+������D��ȷ��
��ѡC��
���� ���⿼���������Һ�����жϣ�Ϊ��Ƶ���㣬��ȷ�ж�����ǿ���ǽⱾ��ؼ���ע�����غ㡢�����غ��������ã��״�ѡ����C���ܶ�ͬѧ������Ϊ������Һ������ʱ������Ũ�ȵ��ڸ��������Ũ�ȶ����´���Ϊ�״��㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
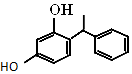 ��������Ѹ�٣��ߴ�ͦ�Σ������а���֮�ƣ���������һ���л���ṹ��ͼ��ʾ������˵��������ǣ�������
��������Ѹ�٣��ߴ�ͦ�Σ������а���֮�ƣ���������һ���л���ṹ��ͼ��ʾ������˵��������ǣ�������| A�� | ���л����������������� | |
| B�� | ����ʽΪC14H14O2 | |
| C�� | 1 mol����������ˮ��Ӧʱ���������2 mol��Br2 | |
| D�� | ���л�������Na2CO3��Һ��Ӧ�������ɵ�������ʹ����ʯ��ˮ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
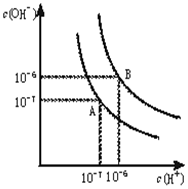 ˮ�ĵ���ƽ����������ͼ��ʾ��
ˮ�ĵ���ƽ����������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
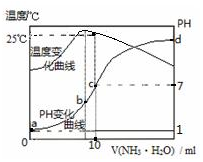
| A�� | n=1.0 | |
| B�� | ˮ�ĵ���̶ȣ�b��c��a��d | |
| C�� | c�㣺c��NH4+��=c��Cl-��=1.0mol•L-1 | |
| D�� | 25��ʱ��NH4Cl��ˮ�ⳣ����Kh��=��n-1����10-7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
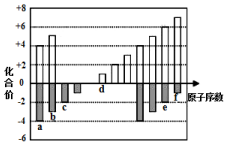 ��ͼ�Dz��ֶ�����Ԫ�ػ��ϼ���ԭ�������Ĺ�ϵͼ����ش��������⣺
��ͼ�Dz��ֶ�����Ԫ�ػ��ϼ���ԭ�������Ĺ�ϵͼ����ش��������⣺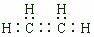 ��
��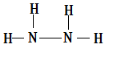 ��A��һ�ֳ��õĻ�ԭ������װ������AgBr���Թ��м���A����Һ���ɹ۲쵽������ڣ��������ݲ�����д���÷�Ӧ�Ļ�ѧ����ʽ4AgBr+N2H4=4Ag+N2��+4HBr��b��c��ԭ����2��4���ɷ���B����֪A��B��Ӧʱ�����������ʣ���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ1��2��
��A��һ�ֳ��õĻ�ԭ������װ������AgBr���Թ��м���A����Һ���ɹ۲쵽������ڣ��������ݲ�����д���÷�Ӧ�Ļ�ѧ����ʽ4AgBr+N2H4=4Ag+N2��+4HBr��b��c��ԭ����2��4���ɷ���B����֪A��B��Ӧʱ�����������ʣ���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ1��2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��84������Һ�е�NaClO��ǿ�����ԣ���ɱ������ | |
| B�� | ʹ��Na2CO3��Һ��ʹ��ϴ�Ӽ��[ϴ�;߸����� | |
| C�� | �����������ս�ɵĽ����մ������¡��������� | |
| D�� | �ơ�����п��ͭ����������������Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ɫƿ�� | B�� | ���ӷ� | C�� | ��ͭ����Ӧ | D�� | ���Ȳ��ֽ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com