
 SiHCl3 + H2��g��
SiHCl3 + H2��g�� Si��s��+ 3HCl��g��
Si��s��+ 3HCl��g��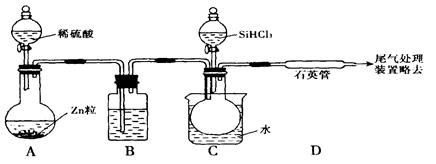



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
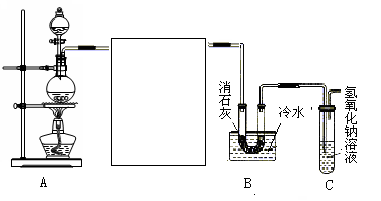
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
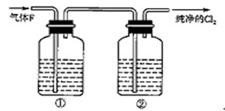
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 д����صĻ�ѧ����ʽ�� ��
д����صĻ�ѧ����ʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ȶ��ռ������壬����ζ�����ռ��� |
| B������ʪ�ĵ���KI��ֽ�ӽ�ƿ�ڣ���ֽ���������ռ��� |
| C������ʪ�ĺ�ɫʯ����ֽ����ƿ�ڣ���ֽ���������ռ��� |
| D������ʪ�ĺ�ɫʯ����ֽ�ӽ�ƿ�ڣ���ֽ���������ռ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
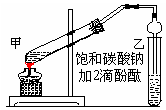
| A���к�������Ҵ��� |
| B���к����Ტ���ղ����Ҵ��� |
| C�����������ڱ���̼������Һ�е��ܽ�ȱ���ˮ�и�С�������ڷֲ������� |
| D�������������ɣ��������ʡ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
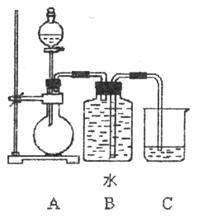
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 CH3CH2Br+NaHSO4 +H2O��
CH3CH2Br+NaHSO4 +H2O��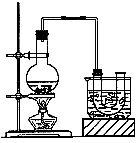
| A��Na2SO3��Һ | B��H2O | C��NaOH��Һ | D��CCl4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������������Ʊ� | B����8%���Ҵ�ˮ��Һ�������� |
| C���Դֱ���������ؽᾧ | D��ͭƬ��Ũ������ȷ�Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com