£Ø1£©»ÆѧŹĒŅ»ĆÅŅŌŹµŃéĪŖ»ł“”µÄѧæĘ£¬»ÆѧŹµŃéŹĒ»ÆѧѧĻ°µÄÖŲŅŖÄŚČŻ£®øł¾ŻÄćÕĘĪÕµÄÖŖŹ¶ÅŠ¶Ļ£¬ĻĀĮŠŹµŃé²Ł×÷µÄĆčŹöÖŠÕżČ·µÄŹĒ
BӢE
BӢE
£®£ØĢīŠ“ĻĀĮŠø÷ĻīŠņŗÅ£©
A£®ČĪŗĪ“ÓŹŌ¼ĮĘæÖŠČ”³öµÄŅ©Ę·£¬ČōÓŠŹ£Óą¾ł²»ÄÜŌŁ·Å»ŲŌŹŌ¼ĮĘæ
B£®ÓĆĻ”ŃĪĖįĻ“µÓŹ¢·Å¹żŹÆ»ŅĖ®µÄŹŌ¼ĮĘæ
C£®ÅäÖĘH
2SO
4ČÜŅŗŹ±£¬æÉĻČŌŚĮæĶ²ÖŠ¼ÓČėŅ»¶ØĢå»żµÄĖ®£¬ŌŁŌŚ½Į°čĻĀĀżĀż¼ÓČėÅØĮņĖį
D£®ø÷·ÅŅ»ÕÅÖŹĮæĻąĶ¬µÄĀĖÖ½ÓŚĢģĘ½µÄĮ½ĶŠÅĢÉĻ£¬½«NaOH¹ĢĢå·ÅŌŚ×óÅĢÖ½ÉĻ³ĘĮæ
E£®Na
2CO
3ČÜŅŗ²»Äܱ£“ęŌŚ“ųÓŠÄ„æŚ²£Į§ČūµÄŹŌ¼ĮĘæÖŠ
F£®½«ÓĆĖ®ČóŹŖµÄPHŹŌÖ½½žČėĻ”ŃĪĖįÖŠ£¬²ā¶ØČÜŅŗµÄPH
£Ø2£©¾Ż±ØµĄ£¬ÄæĒ°Ņ»Š©µŲĒųĖįÓź”¢»Ņö²ŗĶ¹ā»ÆѧŃĢĪķµČĒųÓņŠŌ“óĘųĪŪČ¾Ķ»³ö£¬ŃĻÖŲĶžŠ²ČŗÖŚ½”æµ£¬Ó°Ļģ»·¾³°²Č«£®¶žŃõ»ÆĮņŗĶµŖµÄŃõ»ÆĪļŹĒ“óĘųµÄÖ÷ŅŖĪŪČ¾Īļ£¬¹Ų×¢³ōŃõ²ć”¢·ĄÖ¹ŗĶÖĪĄķ»·¾³ĪŪČ¾ŹĒµ±Ē°»·±£¹¤×÷µÄÖŲŅŖŃŠ¾æÄŚČŻÖ®Ņ»£®
¢ŁĘū³µĪ²ĘųÖ÷ŅŖŗ¬ÓŠCO
2ӢCOӢSO
2”¢NO¼°ĘūÓĶ”¢²ńÓĶµČµ½ĪļÖŹ£¬ÕāÖÖĪ²ĘųŌ½Ą“Ō½³ÉĪŖ³ĒŹŠæÕĘųĪŪČ¾µÄÖ÷ŅŖĄ“Ō“£¬Ęū³µĪ²ĘųÖŠµÄCOĄ“×Ō
ĘūÓĶµÄ²»ĶźČ«Č¼ÉÕ
ĘūÓĶµÄ²»ĶźČ«Č¼ÉÕ
£¬NOĄ“×Ō£ØÓĆ·½³ĢŹ½±ķŹ¾£©
£®
¢ŚæÕĘųÖŠĪ¢ĮæµÄ³ōŃõ¶ŌČĖÓŠŅę£¬ÅØ¶Č¹ż“óŌņŹĒŅ»ÖÖĪŪČ¾ĘųĢ壬³ōŃõ×÷ĪŖĒæŃõ»Æ¼Į£¬¼øŗõÄÜÓėČĪŗĪÉśĪļ×éÖÆ·“Ó¦£®ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ
ABCD
ABCD
£®
A£®Ķ£Ö¹Ź¹ÓĆ·śĄļ°ŗŹĒĪŖĮĖ±£»¤³ōŃõ²ć
B£®¹ā»ÆѧŃĢĪķÓė“óĘų³ōŃõµÄÉś³ÉÓŠ¹Ų
C£®¼ŅÓƵēĘ÷Ļū¶¾¹ńÖŠ²śÉśµÄ³ōŃõÄܹ»øߊ§É±¾śĻū¶¾
D£®³ōŃõÄÜŹ¹ŹŖČóµÄµā»Æ¼ŲŅ»µķ·ŪŹŌÖ½±äĄ¶
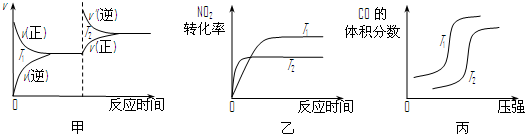
¢ŪŌŚŅ»¶ØĢõ¼žĻĀÓĆ°±æɽ«µŖŃõ»ÆĪļ×Ŗ»ÆĪŖĪŽ¶¾ĘųĢ壮Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬7L NH
3Ē”ŗĆ½«6L NOŗĶNO
2µÄ»ģŗĻĘųĢåĶźČ«×Ŗ»ÆĪŖN
2£¬ŌņŌ»ģŗĻĘųĢåÖŠNOŗĶNO
2µÄĢå»ż±ČŹĒ
1£ŗ3
1£ŗ3
£®


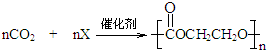 £¬ŌņXµÄ½į¹¹¼ņŹ½ĪŖ
£¬ŌņXµÄ½į¹¹¼ņŹ½ĪŖ
