
ĪŅ¹śæĘѧ¼Ņ·¢ĻÖ½šĖæĢŅĖŲ¶ŌøßÖĀ²”ŠŌĒŻĮ÷øŠ²”¶¾É±ĆšŠ§¹ūĮ¼ŗĆ”£Ä³ÖÖ½šĖæĢŅĖŲµÄ½į¹¹¼ņŹ½ČēĶ¼ĖłŹ¾£ŗ
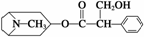
ĻĀĮŠÓŠ¹Ų½šĖæĢŅĖŲµÄĖµ·Ø£ŗ
¢ŁŹōÓŚĢžµÄŃÜÉśĪļ£¬Č¼ÉÕÖ»²śÉśCO2ŗĶH2O””¢ŚæÉŅŌ·¢ÉśČ”“ś”¢¼Ó³É”¢õ„»Æ”¢ĻūČ„µČ·“Ó¦””¢Ū·Ö×ÓŹ½ĪŖC17H23NO3£¬±½»·ÉĻµÄŅ»ĀČČ”“śĪļÓŠ3ÖÖ””¢Ü1 moløĆĪļÖŹ×ī¶ąÄÜŗĶ6 mol H2·¢Éś¼Ó³É·“Ó¦
ĘäÖŠ“ķĪóµÄŹĒ(””””)
A£®¢ŁŗĶ¢Ü B£®¢ŚŗĶ¢Ū C£®¢ŁŗĶ¢Ū D£®¢ŚŗĶ¢Ü

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
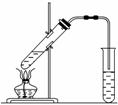 ŹµŃéŹŅÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆÖĘČ”ŅŅĖįŅŅõ„”£
ŹµŃéŹŅÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆÖĘČ”ŅŅĖįŅŅõ„”£
(1)ŌŚŹŌ¹ÜÖŠÅäÖĘŅ»¶Ø±ČĄżµÄŅŅ“¼”¢ŅŅĖįŗĶÅØĮņĖįµÄ»ģŗĻČÜŅŗ£¬Ęä·½·ØŹĒ______________”£
(2)×°ÖĆÖŠĶØÕōĘųµÄµ¼¹ÜÓ¦ÖĆÓŚ±„ŗĶĢ¼ĖįÄĘČÜŅŗµÄŅŗĆęÉĻ¶ų²»ÄܲåČėČÜŅŗÖŠ£¬ÄæµÄŹĒ·ĄÖ¹ČÜŅŗµ¹Īü£¬Ōģ³Éµ¹ĪüµÄŌŅņŹĒ_________________”£
(3)ÅØĮņĖįµÄ×÷ÓĆŹĒ¢Ł________£»¢Ś________”£
(4)±„ŗĶĢ¼ĖįÄĘČÜŅŗµÄ×÷ÓĆŹĒ______________________”£
(5)·“Ó¦Ź±Éś³ÉµÄŅŅĖįŅŅõ„ĆܶȱČĖ®________£¬ÓŠ________ĘųĪ¶”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÖĘČ”°±Ęų²¢Ķź³ÉÅēČŖŹµŃé(Ķ¼ÖŠ¼Š³Ö×°ÖĆ¾łŅŃĀŌČ„)”£
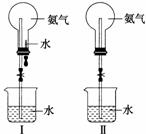
(1)Š“³öŹµŃéŹŅÖĘČ”°±ĘųµÄ»Æѧ·½³ĢŹ½_____________________________________”£
(2)ŹÕ¼Æ°±ĘųÓ¦Ź¹ÓĆ____________________________________________________·Ø£¬ŅŖµĆµ½øÉŌļµÄ°±ĘųæÉŃ”ÓĆ__________×÷øÉŌļ¼Į”£
(3)ÓĆĶ¼¢ńĖłŹ¾×°ÖĆ½ųŠŠÅēČŖŹµŃ飬ÉĻ²æÉÕĘæŅŃ×°ĀśøÉŌļµÄ°±Ęų£¬Ņż·¢ÅēČŖµÄ²Ł×÷ŹĒ______________________________________£¬øĆŹµŃéµÄŌĄķŹĒ________________________”£
(4)Čē¹ūÖ»Ģį¹©ČēĶ¼¢ņĖłŹ¾×°ÖĆ£¬ĒėĖµĆ÷Ņż·¢ÅēČŖµÄ·½·ØŹĒ____________________
____________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«NaCl”¢KAl(SO4)2”¢FeCl2”¢Fe2(SO4)3”¢Mg(NO3)2ĪåÖÖČÜŅŗ£¬Ö»ÓĆŅ»ÖÖŹŌ¼ĮĶعżŹµŃé¾ĶÄܼÓŅŌĒų±š£¬ÕāÖÖŹŌ¼ĮŹĒ(””””)
A£®KSCN B£®BaCl2 C£®NaOH D£®NaCl
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
µē×Ó¹¤Ņµ³£ÓĆ30%µÄFeCl3ČÜŅŗøÆŹ“¶ĘŌŚ¾ųŌµ°åÉĻµÄĶ²£¬ÖĘŌģÓ”Ė¢µēĀ·°å”£
(1)¼ģŃéČÜŅŗÖŠFe3£«µÄŹŌ¼ĮŹĒ________”£
(2)Š“³öFeCl3ČÜŅŗÓė½šŹōĶ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ_________________________”£
(3)ij¹¤³ĢŹ¦ĪŖĮĖ“ÓŹ¹ÓĆ¹żµÄøÆŹ“·ĻŅŗÖŠ»ŲŹÕĶ£¬²¢ÖŲŠĀ»ńµĆ“æ¾»µÄFeCl3ČÜŅŗ£¬×¼±øÓĆČēĶ¼ĖłŹ¾²½Öč£ŗ
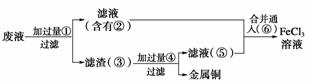
A£®Š“³öÉĻŹöŹµŃéÖŠ¼ÓČė»ņÉś³ÉµÄÓŠ¹ŲĪļÖŹµÄ»ÆѧŹ½£ŗ
¢Ł________£»¢Ś________£»¢Ū________£»¢Ü________£»¢Ż________£»¢Ž________”£
B£®Š“³öĻĀĮŠ¹ż³ĢÖŠµÄĄė×Ó·½³ĢŹ½£ŗ
¢Ł”ś¢Ś________________________________________________________________________£»
¢Ż£«¢Ž________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŻĘĘ·“¼æÉ×÷ĪŖĻū¶¾¼Į”¢æ¹Ńõ»Æ¼Į”¢Ņ½Ņ©ŗĶČܼĮ”£ŗĻ³É¦ĮŻĘĘ·“¼GµÄĀ·ĻßÖ®Ņ»ČēĻĀ£ŗ
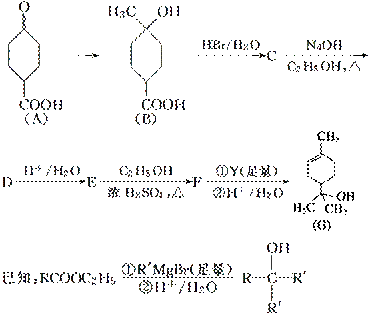
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)AĖłŗ¬¹ŁÄÜĶŵÄĆū³ĘŹĒ__________”£
(2)A“ß»ÆĒā»ÆµĆZ(C7H12O3)£¬Š“³öZŌŚŅ»¶ØĢõ¼žĻĀ¾ŪŗĻ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ________________________________________________________________________”£
(3)BµÄ·Ö×ÓŹ½ĪŖ__________£»Š“³öĶ¬Ź±Āś×ćĻĀĮŠĢõ¼žµÄBµÄĮ“דĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ________________________________________________________________________”£
¢ŁŗĖ“Ź²ÕńĒāĘ×ÓŠ2øöĪüŹÕ·å
¢ŚÄÜ·¢ÉśŅų¾µ·“Ó¦
(4)BØD”śC”¢EØD”śFµÄ·“Ó¦ĄąŠĶ·Ö±šĪŖ__________”¢______________”£
(5)CØD”śDµÄ»Æѧ·½³ĢŹ½ĪŖ_________________________________________________”£
(6)ŹŌ¼ĮYµÄ½į¹¹¼ņŹ½ĪŖ___________________________________________________”£
(7) Ķعż³£ĪĀĻĀµÄ·“Ó¦£¬Ēų±šE”¢FŗĶGµÄŹŌ¼ĮŹĒ_____________________________
ŗĶ__________”£
(8)GÓėH2O“ß»Æ¼Ó³ÉµĆ²»ŗ¬ŹÖŠŌĢ¼Ō×Ó(Į¬ÓŠ4øö²»Ķ¬Ō×Ó»ņŌ×ÓĶŵÄĢ¼Ō×Ó½ŠŹÖŠŌĢ¼Ō×Ó)µÄ»ÆŗĻĪļH£¬Š“³öHµÄ½į¹¹¼ņŹ½£ŗ__________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
(””””)”£
A£®½ūÖ¹Ź¹ÓĆĖÄŅŅ»łĒ¦×÷ĘūÓĶæ¹±¬Õš¼Į£¬æɼõÉŁĘū³µĪ²ĘųĪŪČ¾
B£®¾ŪŅŅĻ©æÉ·¢Éś¼Ó³É·“Ó¦
C£®ĆŗµÄøÉĮóŗĶŹÆÓĶµÄ·ÖĮó¾łŹō»Æѧ±ä»Æ
D£®ŹÆÓĶ·ÖĮóæÉ»ńµĆŅŅĻ©”¢±ūĻ©ŗĶ¶”¶žĻ©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŌŅŅ“¼ĪŖŌĮĻ£¬ÓĆĻĀŹö6ÖÖĄąŠĶµÄ·“Ó¦Ą“ŗĻ³ÉŅŅ¶žĖįŅŅ¶žõ„(½į¹¹¼ņŹ½ĪŖ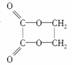 )£¬ÕżČ·µÄĖ³ŠņŹĒ(””””)
)£¬ÕżČ·µÄĖ³ŠņŹĒ(””””)
¢ŁŃõ»Æ””¢ŚĻūČ„””¢Ū¼Ó³É””¢Üõ„»Æ””¢ŻĖ®½ā””¢Ž¼Ó¾Ū
A£®¢Ł¢Ż¢Ś¢Ū¢Ü B£®¢Ł¢Ś¢Ū¢Ü¢Ż
C£®¢Ś¢Ū¢Ż¢Ł¢Ü D£®¢Ś¢Ū¢Ż¢Ł¢Ž
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Īķö²ŃĻÖŲÓ°ĻģČĖĆĒµÄÉś»īÓė½”æµ”£Ä³µŲĒųµÄĪķö²ÖŠæÉÄÜŗ¬ÓŠČēĻĀæÉČÜŠŌĪŽ»śĄė×Ó£ŗNa£«”¢NH ”¢Mg2£«”¢Al3£«”¢SO
”¢Mg2£«”¢Al3£«”¢SO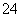 ”¢NO
”¢NO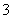 ”¢Cl£”£Ä³Ķ¬Ń§ŹÕ¼ÆĮĖøƵŲĒųµÄĪķö²£¬¾±ŲŅŖµÄŌ¤“¦ĄķŗóµĆŹŌŃłČÜŅŗ£¬Éč¼Ę²¢Ķź³ÉĮĖČēĻĀŹµŃé£ŗ
”¢Cl£”£Ä³Ķ¬Ń§ŹÕ¼ÆĮĖøƵŲĒųµÄĪķö²£¬¾±ŲŅŖµÄŌ¤“¦ĄķŗóµĆŹŌŃłČÜŅŗ£¬Éč¼Ę²¢Ķź³ÉĮĖČēĻĀŹµŃé£ŗ
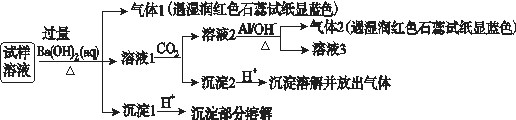
ŅŃÖŖ£ŗ3NO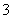 £«8Al£«5OH££«2H2O=3NH3”ü£«8AlO
£«8Al£«5OH££«2H2O=3NH3”ü£«8AlO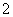
øł¾ŻŅŌÉĻµÄŹµŃé²Ł×÷ÓėĻÖĻó£¬øĆĶ¬Ń§µĆ³öµÄ½įĀŪ²»ÕżČ·µÄŹĒ(””””)
A£®ŹŌŃłÖŠæĻ¶Ø“ęŌŚNH ”¢Mg2£«”¢SO
”¢Mg2£«”¢SO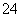 ŗĶNO
ŗĶNO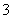 B£®ŹŌŃłÖŠæÉÄÜ“ęŌŚNa£«”¢Cl£
B£®ŹŌŃłÖŠæÉÄÜ“ęŌŚNa£«”¢Cl£
C£®øĆĪķö²ÖŠæÉÄÜ“ęŌŚNaNO3”¢NH4ClŗĶMgSO4 D£®ŹŌŃłÖŠŅ»¶Ø²»ŗ¬Al3£«
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com