��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ�
��1������ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H
2��g��+CO��g��?CH
3OH��g������H=-90.8kJ?mol
-1��2CH
3OH��g��?CH
3OCH
3��g��+H
2O��g������H=-23.5kJ?mol
-1��CO��g��+H
2O��g��?CO
2��g��+H
2��g������H=-41.3kJ?mol
-1�ܷ�Ӧ��3H
2��g��+3CO��g��?CH
3OCH
3��g��+CO
2 ��g���ġ�H=
-246.4KJ/mol
-246.4KJ/mol
��
��2����֪��Ӧ��2CH
3OH��g��?CH
3OCH
3��g��+H
2O��g��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH
3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
| ���� |
CH3OH |
CH3OCH3 |
H2O |
| Ũ��/��mol?L-1�� |
0.44 |
0.6 |
0.6 |
�ٱȽϴ�ʱ�����淴Ӧ���ʵĴ�С��v
����
��
v
�� �����������������=������
��������CH
3OH��10min��Ӧ�ﵽƽ�⣬��ʱc��CH
3OH��=
0.04mol/L
0.04mol/L
����ʱ���ڷ�Ӧ����v��CH
3OH��=
0.16mol/L?min
0.16mol/L?min
��
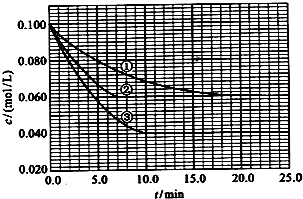
��3������Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊc��A��=0.100mol/L��c��B��=0.200mol/L��c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ��
��ش��������⣺
��ٱȽϣ��ں͢۷ֱ���ı�һ�ַ�Ӧ���������ı���������жϵ������ǣ�
��
�Ӵ������ﵽƽ���ʱ�����̣�ƽ��ʱA��Ũ�Ȳ���
�Ӵ������ﵽƽ���ʱ�����̣�ƽ��ʱA��Ũ�Ȳ���
��
��
�¶����ߣ��ﵽƽ���ʱ�����̣�ƽ��ʱA��Ũ�ȼ�С
�¶����ߣ��ﵽƽ���ʱ�����̣�ƽ��ʱA��Ũ�ȼ�С
��
�÷�Ӧ�ġ�H
��
��
0�����������
�����¶ȷ�Ӧ����Ӧ���ʷ�Ӧ�����ȷ�Ӧ
�����¶ȷ�Ӧ����Ӧ���ʷ�Ӧ�����ȷ�Ӧ
��




 ��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ�
��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ�