
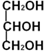
 �����������ᣨCH3CH2COOH�����������ķ�Ӧ����ʽ��
�����������ᣨCH3CH2COOH�����������ķ�Ӧ����ʽ��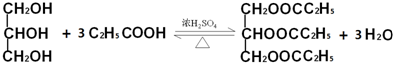
 �������ݲ������Ҳ����������ȼ������ṹΪCH3CH2OH����������������ΪCH3OCH3��
�������ݲ������Ҳ����������ȼ������ṹΪCH3CH2OH����������������ΪCH3OCH3�� ���� ��1�����봼����������Ӧʱ�������ǻ������⣻
��2��Cl2��ClO2�������з���������ԭ��Ӧ����Ԫ�ػ��ϼ۾����͵�-1�ۣ�����ת�Ƶ�������Ƚ��м��㣻
��3���屽�������������ʿ���ѡ������������Һ��ȥ��
��4��Ũ������ͭ��Ӧ��������ͭ�����������ˮ��������Ũ��������Ժ�ǿ�����ԣ�
��5��笠��ļ��鷽��Ϊ��ǿ����Ȳ�������������ʪ��ĺ�ɫʯ����ֽ�����Թܿڼ��鰱����
��6��������������������NO��ˮ������Ϊ��ɫ����С��������泥�
��7������ʽΪC2H6O���л���ṹ�����Ǵ������ѣ����ݴ��ǻ����Խ����Ʒ�Ӧ�����������Ѳ���Ӧ����������
��� �⣺��1�����봼����������Ӧʱ�������ǻ������⣬��Ӧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��2��Cl2��ClO2�������з���������ԭ��Ӧ����Ԫ�ػ��ϼ۾����͵�-1�ۣ�����ת�Ƶ�������ȣ�0.01mol��Cl2������Ӧת��0.02mol���ӣ����б����VmL��ClO2��Ҫת����ͬ��Ŀ�ĵ��ӣ���$\frac{V��1{0}^{-3}}{22.4}��5=0.02$����V=89.6mL��
�ʴ�Ϊ��89.6��
��3���屽�������������ʿ���ѡ������������Һ��ȥ����Ӧ����ʽΪBr2+2NaOH=NaBr+NaBrO+H2O��
�ʴ�Ϊ��Br2+2NaOH=NaBr+NaBrO+H2O��
��4��Ũ������ͭ��Ӧ��������ͭ�����������ˮ��������Ũ��������Ժ�ǿ�����ԣ�
�ʴ�Ϊ�����Ժ�ǿ�����ԣ�
��5��笠��ļ��鷽��Ϊ��ǿ����Ȳ�������������ʪ��ĺ�ɫʯ����ֽ�����Թܿڼ��鰱��������ֽ������Ϊ�����Һ����֮���ǣ�
�ʴ�Ϊ��ȡ����������Һ���Թ��У�����ŨNaOH��Һ�������ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ������Ϊ�����Һ����֮���ǣ�
��6��������������������NO��ˮ����ӦΪ4NH3+5O2 $\frac{\underline{����}}{��}$4NO+6H2O��������ͨ����죬����һ�������������ɶ�������������ˮ�������ᣬ�����백����Ӧ���ɰ�ɫ����С����NH4NO3��
�ʴ�Ϊ��4NH3+5O2 $\frac{\underline{����}}{��}$4NO+6H2O�� NH4NO3��
��7������ʽΪC2H6O���л���ṹ�����Ǵ������ѣ����ǻ������Ʒ�Ӧ�����������Ѳ��ܷ�Ӧ����װ�ÿ����Ϊ ���������ݲ������Ҳ����������ȼ������ṹΪCH3CH2OH����������������ΪCH3OCH3��
���������ݲ������Ҳ����������ȼ������ṹΪCH3CH2OH����������������ΪCH3OCH3��
�ʴ�Ϊ�� �������ݲ������Ҳ����������ȼ������ṹΪCH3CH2OH����������������ΪCH3OCH3��
�������ݲ������Ҳ����������ȼ������ṹΪCH3CH2OH����������������ΪCH3OCH3��
���� �����Ϊ�ۺϣ�������������Ӧ��ԭ����������ԭ���㡢���Ĵ�������笠��ļ��顢�л���ļ����֪ʶ��Ҫȥѧ��������ʵ�Ļ���֪ʶ����Ŀ�ѶȲ���ע����ϸ���⣮


 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+ | B�� | SO42- | C�� | S 2- | D�� | NH4+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �� | B�� | ������������ͭ | ||
| C�� | ������������ͭ��Һ | D�� | ��ˮ | ||
| E�� | ������������������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
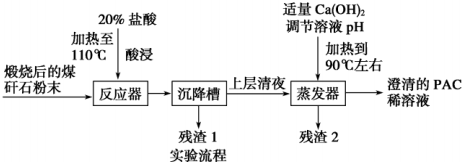
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
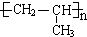 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
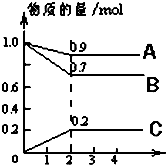 ij�¶�ʱ����2L�ܱ������У�A��B��C�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ����ͼ�����ݷ�����
ij�¶�ʱ����2L�ܱ������У�A��B��C�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ����ͼ�����ݷ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
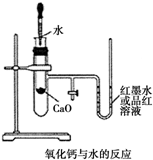 ��ͼ��ijͬѧ��Ƶķ��ȷ�Ӧ�Ĺ۲�װ�ã���ʵ������������£�
��ͼ��ijͬѧ��Ƶķ��ȷ�Ӧ�Ĺ۲�װ�ã���ʵ������������£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������� | B�� | ����� | C�� | ���ӻ����� | D�� | �л��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com