
 A”¢B”¢C”¢DŹĒ¶ĢÖÜĘŚŌŖĖŲŠĪ³ÉµÄĖÄÖÖĘųĢåµ„ÖŹ£®E”¢F¾łĪŖĘųĢ壬ĒŅFĪŖŗģ×ŲÉ«£®ÓŠ¹ŲµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£Ø·“Ó¦Ģõ¼ž¾łŅŃĀŌČ„£©£®
A”¢B”¢C”¢DŹĒ¶ĢÖÜĘŚŌŖĖŲŠĪ³ÉµÄĖÄÖÖĘųĢåµ„ÖŹ£®E”¢F¾łĪŖĘųĢ壬ĒŅFĪŖŗģ×ŲÉ«£®ÓŠ¹ŲµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾£Ø·“Ó¦Ģõ¼ž¾łŅŃĀŌČ„£©£®| c(H+) |
| c(OH-) |
 NH4++OH-æɵĆÓÉ°±Ė®µēĄė³öµÄc£ØNH4+£©=1”Į10-3 mol?L-1£¬B“ķ£®ÓÉĖ®µēĄė³öµÄc£ØH+£©=c£ØOH-£©=1”Į10-11mol?L-1£¬CÕżČ·£®Ń”ĻīDĻŌČ»ŹĒÕżČ·£®Ń”ĻīEæ¼ĀĒµ½°±Ė®ŹĒČõµē½āČÜŅŗ£¬¹Ź¼ÓĖ®Ļ”ŹĶ100±¶ŗó£¬ČÜŅŗµÄpH“óÓŚ9£®
NH4++OH-æɵĆÓÉ°±Ė®µēĄė³öµÄc£ØNH4+£©=1”Į10-3 mol?L-1£¬B“ķ£®ÓÉĖ®µēĄė³öµÄc£ØH+£©=c£ØOH-£©=1”Į10-11mol?L-1£¬CÕżČ·£®Ń”ĻīDĻŌČ»ŹĒÕżČ·£®Ń”ĻīEæ¼ĀĒµ½°±Ė®ŹĒČõµē½āČÜŅŗ£¬¹Ź¼ÓĖ®Ļ”ŹĶ100±¶ŗó£¬ČÜŅŗµÄpH“óÓŚ9£® NH3?H2O+H+£¬ļ§øłĄė×ÓĖ®½ā¶ųŹ¹ČÜŅŗĻŌĖįŠŌ£¬
NH3?H2O+H+£¬ļ§øłĄė×ÓĖ®½ā¶ųŹ¹ČÜŅŗĻŌĖįŠŌ£¬ NH3?H2O+H+£»
NH3?H2O+H+£»| c(H+) |
| c(OH-) |
 NH4++OH-æɵĆÓÉ°±Ė®µēĄė³öµÄc£ØNH4+£©=1”Į10-3 mol?L-1£¬¹ŹB“ķĪó£»
NH4++OH-æɵĆÓÉ°±Ė®µēĄė³öµÄc£ØNH4+£©=1”Į10-3 mol?L-1£¬¹ŹB“ķĪó£»

 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģÄžĻÄŅų“ØŅ»ÖŠøßČżµŚČż“ĪÄ£Äāæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
”¾»ÆѧŅ»Ń”ŠŽ3£ŗĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ£Ø15·Ö£©
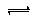
ĻÖÓŠĮłÖÖŌŖĖŲ£¬ĘäÖŠA”¢B”¢C”¢DĪŖ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬E”¢FĪŖµŚĖÄÖÜĘŚŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó”£Ēėøł¾ŻĻĀĮŠĻą¹ŲŠÅĻ¢£¬»Ų“šĪŹĢā£®
| AŌŖĖŲŌ×ÓµÄŗĖĶāpµē×Ó×ÜŹż±Čsµē×Ó×ÜŹżÉŁ1 |
| BŌŖĖŲŌ×ÓŗĖĶāsµē×Ó×ÜŹżÓėpµē×Ó×ÜŹżĻąµČ£¬ĒŅ²»ÓėAŌŖĖŲŌŚĶ¬Ņ»ÖÜĘŚ |
| CŌ×ÓŗĖĶāĖłÓŠp¹ģµĄČ«Āś»ņ°ėĀś |
| DŌŖĖŲµÄÖ÷×åŠņŹżÓėÖÜĘŚŹżµÄ²īĪŖ4 |
| EŹĒĒ°ĖÄÖÜĘŚÖŠµēøŗŠŌ×īŠ”µÄŌŖĖŲ |
| FŌŚÖÜĘŚ±ķµÄµŚĘßĮŠ |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğŗÓ±±Ź”ŗāĖ®ŹŠµŚŹ®ĖÄ֊ѧø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
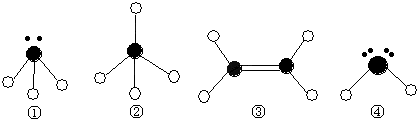
ĻÖÓŠĮłÖÖŌŖĖŲ£¬ĘäÖŠA”¢B”¢C”¢DĪŖ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬E”¢FĪŖµŚĖÄÖÜĘŚŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó”£Ēėøł¾ŻĻĀĮŠĻą¹ŲŠÅĻ¢£¬»Ų“šĪŹĢā”£
| AŌŖĖŲŌ×ÓµÄŗĖĶāpµē×Ó×ÜŹż±Čsµē×Ó×ÜŹżÉŁ1 |
| BŌŖĖŲŌ×ÓŗĖĶāsµē×Ó×ÜŹżÓėpµē×Ó×ÜŹżĻąµČ£¬ĒŅ²»ÓėAŌŖĖŲŌŚĶ¬Ņ»ÖÜĘŚ |
| CŌ×ÓŗĖĶāĖłÓŠp¹ģµĄČ«Āś»ņ°ėĀś |
| DŌŖĖŲµÄÖ÷×åŠņŹżÓėÖÜĘŚŹżµÄ²īĪŖ4 |
| EŹĒĒ°ĖÄÖÜĘŚÖŠµēøŗŠŌ×īŠ”µÄŌŖĖŲ |
| FŌŚÖÜĘŚ±ķµÄµŚĘßĮŠ |
 £¬Ī„±³ĮĖ ŌĄķ”£
£¬Ī„±³ĮĖ ŌĄķ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğĞĻÄøßČżµŚČż“ĪÄ£Äāæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
”¾»ÆѧŅ»Ń”ŠŽ3£ŗĪļÖŹ½į¹¹ÓėŠŌÖŹ”æ£Ø15·Ö£©
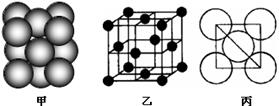
ĻÖÓŠĮłÖÖŌŖĖŲ£¬ĘäÖŠA”¢B”¢C”¢DĪŖ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬E”¢FĪŖµŚĖÄÖÜĘŚŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó”£Ēėøł¾ŻĻĀĮŠĻą¹ŲŠÅĻ¢£¬»Ų“šĪŹĢā£®
|
AŌŖĖŲŌ×ÓµÄŗĖĶāpµē×Ó×ÜŹż±Čsµē×Ó×ÜŹżÉŁ1 |
|
BŌŖĖŲŌ×ÓŗĖĶāsµē×Ó×ÜŹżÓėpµē×Ó×ÜŹżĻąµČ£¬ĒŅ²»ÓėAŌŖĖŲŌŚĶ¬Ņ»ÖÜĘŚ |
|
CŌ×ÓŗĖĶāĖłÓŠp¹ģµĄČ«Āś»ņ°ėĀś |
|
DŌŖĖŲµÄÖ÷×åŠņŹżÓėÖÜĘŚŹżµÄ²īĪŖ4 |
|
EŹĒĒ°ĖÄÖÜĘŚÖŠµēøŗŠŌ×īŠ”µÄŌŖĖŲ |
|
FŌŚÖÜĘŚ±ķµÄµŚĘßĮŠ |
£Ø1£©A»łĢ¬Ō×ÓÖŠÄÜĮæ×īøߵĵē×Ó£¬Ęäµē×ÓŌĘŌŚæÕ¼äÓŠ øö·½Ļņ£¬Ō×Ó¹ģµĄ³Ź
ŠĪ
£Ø2£©Ä³Ķ¬Ń§øł¾ŻÉĻŹöŠÅĻ¢£¬Ėł»µÄBµē×ÓÅŲ¼Ķ¼ČēĶ¼
Ī„±³ĮĖ ŌĄķ”£
£Ø3£©FĪ»ÓŚ ×å Ēų£¬Ę仳Ģ¬Ō×ÓÓŠ ÖÖŌĖ¶ÆדĢ¬”£
£Ø4£©CD3 ÖŠŠÄŌ×ÓµÄŌӻƷ½Ź½ĪŖ £¬ÓĆ¼Ū²ćµē×Ó¶Ō»„³āĄķĀŪĶĘ²āĘä·Ö×Óæռ乹ŠĶĪŖ £®¼ģŃéEŌŖĖŲµÄ·½·ØŹĒ
£Ø5£©Čōij½šŹōµ„ÖŹ¾§ĢåÖŠŌ×ӵĶѻż·½Ź½ČēĻĀĶ¼¼×ĖłŹ¾£¬Ę侧°ūĢŲÕ÷ČēĻĀĶ¼ŅŅĖłŹ¾£¬Ō×ÓÖ®¼äĻą»„Ī»ÖĆ¹ŲĻµµÄĘ½ĆęĶ¼ČēĻĀĶ¼±ūĖłŹ¾”£Ōņ¾§°ūÖŠøĆŌ×ÓµÄÅäĪ»ŹżĪŖ £¬øƵ„ÖŹ¾§ĢåÖŠŌ×ӵĶѻż·½Ź½ĪŖĖÄÖÖ»ł±¾¶Ń»ż·½Ź½ÖŠµÄ £®

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø1£©Š“³ö¢ŚÖŠŃĪµÄĖ®ČÜŅŗĻŌĖįŠŌµÄŌŅņ£ØŠ“³öĄė×Ó·½³ĢŹ½£©_____________________£»
£Ø2£©ŅŃÖŖA”¢B”¢C”¢DæÉ×é³ÉŌ×ÓøöŹż±Č5”Ć1”Ć1”Ć3µÄ»ÆŗĻĪļ£¬Š“³öøĆ»ÆŗĻĪļÓė×ćĮæNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ__________________________”£
£Ø3£©”°³¤Õ÷2ŗÅ”±ŌĖŌŲ»š¼żĖłÓĆČ¼ĮĻ¾ĶŹĒCµÄĒā»ÆĪļ±»Č”“śŗóµÄ²śĪļ£ØĻą¶Ō·Ö×ÓÖŹĮæ60£¬ĘäÖŠBµÄÖŹĮæ·ÖŹżĪŖ0.40£¬AµÄÖŹĮæ·ÖŹżĪŖ0.1333£¬ĘäÓąĪŖC£©£¬¶ųC”¢DŠĪ³ÉµÄŌ×ÓøöŹż±Č1”Ć2µÄ»ÆŗĻĪļ£ØĻą¶Ō·Ö×ÓÖŹĮæ92£©¾ĶŹĒøĆČ¼ĮĻµÄŃõ»Æ¼Į”£ĒŅŅŃÖŖĖüĆĒÖ®¼ä·¢ÉśŃõ»Æ»¹Ō·“Ó¦ŗóÉś³ÉµÄ²śĪļ¶Ō»·¾³ĪŽĪŪČ¾”£ŌņøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_____________________”£
£Ø4£©CŌŖĖŲµÄĘųĢ¬Ēā»ÆĪļÄÜ»¹ŌCuO£¬Éś³ÉCu”¢Cµ„ÖŹ¼°AÓėDŠĪ³ÉµÄ»ÆŗĻĪļ£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com