
| A�� | �����Ӿ���ڷ��Ӿ���۷��Ӿ���ܽ������� | |
| B�� | ��ԭ�Ӿ���ڷ��Ӿ���۷��Ӿ���ܽ������� | |
| C�� | �����Ӿ���ڷ��Ӿ���۽�������ܽ������� | |
| D�� | ��ԭ�Ӿ�������Ӿ���۷��Ӿ���ܷ��Ӿ��� |
���� ���Ӿ��������������ӹ��ɣ����Ӽ����������ǿ�����нϸߵ��۵㡢�е㡢Ӳ�ȴ�����״̬��ˮ��Һ�ܵ�������ʣ�
��� �⣺���۵�1070�棬������ˮ��ˮ��Һ���磬�������Ӿ�����ص㣬�ʢ�Ϊ���Ӿ��壻
���۵�Ϊ10.31�棬�۵�ͣ����Ϸ��Ӿ�����ص㣬Һ̬�����磬������Һ̬ʱ��ֻ���ڷ��ӣ�û�����ӣ�ˮ��Һ�ܵ��磬����ˮ������ˮ���ӵ������£�����������ƶ������ӣ��ʢ�Ϊ���Ӿ��壻
��������CS2���۵�112.8�棬�е�444.6�棬���ڷ��Ӿ�����ص㣬�ʢ�Ϊ���Ӿ��壻
�ܽ������۵�Ϊ97.81�棬���������硢�ܶ�0.97g/cm3��Ϊ����������ص㣬�ʢ�Ϊ�������壻
��ѡA��
���� ������Ҫ���������Ӿ�����������ʣ�����ʱ���������Ӿ����۷е�������������ʼ��ɽ���ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ�Ӱ뾶�������� | B�� | ���Ӱ뾶�������� | ||
| C�� | ���ʵ��ܶ����������� | D�� | ��������ļ���������ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������+5�� | B�� | �����Ϊ-2�� | ||
| C�� | ��Ԫ��λ�ڵڶ����� | D�� | ��Ԫ�����ڢ�A��Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 78% | B�� | 22% | C�� | 14% | D�� | 13% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO2 | B�� | CaCl2 | C�� | SiO2 | D�� | Na2O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ���������� | Fe3+ | Al3+ | Mn2+ | Mg2+ |
| ��ȫ����ʱ��PHֵ | 3.2 | 5.2 | 10.4 | 12.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ����NaOH���� | B�� |  ����һ�����ʵ���Ũ��ϡ���� | ||
| C�� |  ��ȡ������Fe��OH��3���� | D�� |  ��H2SO4����Һ�ζ�NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
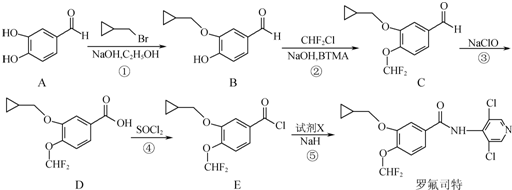
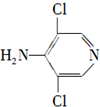 ��
��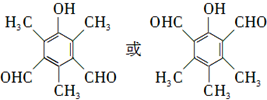 �ȣ�
�ȣ�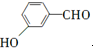 ��CH3CH2OHΪԭ���Ʊ�
��CH3CH2OHΪԭ���Ʊ�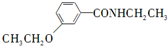 �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com