
��10�֣�ij�����о�С�飬�ú��н϶����ʵ�ͭ�ۣ�ͨ����ͬ�Ļ�ѧ��Ӧ��ȡ����������Ƶ�ʵ�����Ϊ��
��1����ͭ�к��д������л���ɲ������յķ�����ȥ�л������ʱ������������ �ϣ����������������ı�����룬��ͬ����ȡ������Ӧʹ�� �����պ������Ӧ���� ����ȴ������ֱ�ӷ��������ϡ�
����������a�������� b��ʯ���� c�������� d�������� e������ǯ f���Թܼ�
��2����ͭ�����պ�õ��IJ���������ͭ������ͭ�Ļ���������ȡ���������պ�������ͭ�Ŀ���ԭ���� ��
a�����չ����в�������ͭ����ԭ b�����ղ����ͭδ����ȫ����
c������ͭ�ڼ��ȹ����зֽ�����ͭ d����������ͭ������������
��3��ͨ��;��IIʵ���ô�������ͭ��ȡ������������е�ʵ��������裺���ܡ�����ͨ���������ˡ�_____����ȴ�ᾧ�� ����Ȼ���
��4���ɴ�������ͭͨ������;����ȡ��������;��I��ȣ�;��II�����Ե������ŵ��ǣ�
�� ��
��5���ڲⶨ���õ���(CuSO4��xH2O)�нᾧˮxֵ��ʵ���У����ⶨ���xֵƫ�ߣ����ܵ�ԭ���� ��
a�������¶ȹ��� b����������Ŀ����ϴ�
c�����Ⱥ���ڿ�������ȴ d���������岿�ַ绯

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣������������壨FeSO4��7H2O����ҽҩ������Ѫ����ij�����о�С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�
��ش��������⣺
��1��֤���������Һ�к���Fe2+�ķ������ȵμ�KSCN��Һ���ٵμ� ���ù��̵�����Ϊ�� ��
��2������ڼ������H2O2��Ŀ�ģ� ��
��3��������з�Ӧ�����ӷ���ʽ�� ��
��4���������һϵ�д����IJ������裺���ˡ� �����ա� ��������
��5����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص����� g��
��6����С����Щͬѧ��Ϊ��KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ����5Fe2++MnO4��+8H+��5Fe3++Mn2++4H2O��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ���������ձ�����ͷ�ι��⣬���� ��
������ʵ���е�KMnO4��Һ��Ҫ�ữ�������ữ������__ __��
a��ϡ���� b��ϡ���� c��ϡ���� d��Ũ����
�۵ζ����յ�ʱ����ɫΪ ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�찲��ʡ��ʦ���и�����ѧ�ڵ��Ĵ�ģ�⿼�Ի�ѧ�Ծ� ���ͣ�ʵ����
��16�֣������������壨FeSO4��7H2O����ҽҩ������Ѫ����ij�����о�С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�
 ��ش��������⣺
��ش��������⣺
��1��֤���������Һ�к���Fe2+�ķ������ȵμ�KSCN��Һ���ٵμ� ���ù��̵�����Ϊ�� ��
��2������ڼ������H2O2��Ŀ�ģ� ��
��3��������з�Ӧ�����ӷ���ʽ�� ��
��4���������һϵ�д����IJ������裺���ˡ� �����ա� ��������
��5����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص����� g��
��6����С����Щͬѧ��Ϊ��KMnO4��Һ�ζ�Ҳ�ܽ��� ��Ԫ�غ����IJⶨ����5Fe2++MnO4��+8H+��5Fe3++Mn2++4H2O��
��Ԫ�غ����IJⶨ����5Fe2++MnO4��+8H+��5Fe3++Mn2++4H2O��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ���������ձ�����ͷ�ι��⣬���� ��
������ʵ���е�KMnO4��Һ��Ҫ�ữ�������ữ������__ __��
a��ϡ���� b��ϡ���� c��ϡ���� d��Ũ����
�۵ζ����յ�ʱ����ɫΪ ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ������ѧ�ڵ��Ĵ�ģ�⿼�Ի�ѧ�Ծ� ���ͣ�ʵ����
��16�֣������������壨FeSO4��7H2O����ҽҩ������Ѫ����ij�����о�С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�
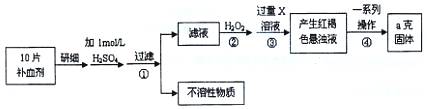
��ش��������⣺
��1��֤���������Һ�к���Fe2+�ķ������ȵμ�KSCN��Һ���ٵμ� ���ù��̵�����Ϊ�� ��
��2������ڼ������H2O2��Ŀ�ģ� ��
��3��������з�Ӧ�����ӷ���ʽ�� ��
��4���������һϵ�д����IJ������裺���ˡ� �����ա� ��������
��5����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص����� g��
��6����С����Щͬѧ��Ϊ��KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ����5Fe2++MnO4��+8H+��5Fe3++Mn2++4H2O��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ���������ձ�����ͷ�ι��⣬���� ��
������ʵ���е�KMnO4��Һ��Ҫ�ữ�������ữ������__ __��
a��ϡ���� b��ϡ���� c��ϡ���� d��Ũ����
�۵ζ����յ�ʱ����ɫΪ ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ϻ��и�����һ�ο��Ի�ѧ�Ծ� ���ͣ�ʵ����
�����������壨FeSO4��7H2O����ҽҩ������Ѫ����ij�����о�С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�

��ش��������⣺
��1��֤���������Һ�к���Fe2+�ķ������ȵμ�KSCN��Һ���ٵμ� ���ù��̵�����Ϊ�� ��
��2������ڼ������H2O2��Ŀ�ģ� ��
��3��������з�Ӧ�����ӷ���ʽ�� ��
��4���������һϵ�д����IJ������裺���ˡ� �����ա� ��������
��5����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص����� g��
��6����С����Щͬѧ��Ϊ��KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ��
��5Fe2++MnO��4+8H+��5Fe3++Mn2++4H2O��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ���������ձ�����ͷ�ι��⣬����
������ʵ���е�KMnO4��Һ��Ҫ�ữ�������ữ������____��
a��ϡ���� b��ϡ���� c��ϡ���� d��Ũ����
�۵ζ����յ�ʱ����ɫΪ ɫ��
��7��������ÿ��Ӧ����14mg���ҵ��������о���������ʳ����ȫ��ͨ�����ú�FeSO4��7H2O��Ƭ��������������������ÿ������ú� mg FeSO4��7H2O��Ƭ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com