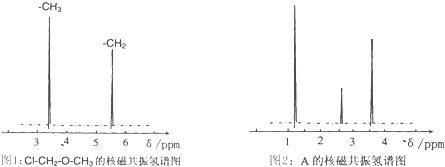
���𰸡�
��������1�����������غ������
��2������ʵ��ʽ����Է��������������ʽ��
��3������ͬ���칹������д��
��4�����ݵ�Ч�������
��5�����ݴ��ܱ������������Һ��������ȩ��д��ѧ����ʽ��
��6��������ϩ�ܷ����Ӿ۷�Ӧ���ɾ���ϩ��
��7��������ϩ�����Ȼ��ⷢ���Ӿ۷�Ӧ���������飻
��8�����ݴ��������������ᣬ��ʹ��ܷ���������Ӧ��
����⣺��1��5.4gH
2O����0.3mol����H��0.6mol��8.8gCO
2��0.2mol����C��0.2mol��6.72LO
2��0.3mol��0.6molH����0.15molO
2��0.2molC����0.2molO
2����O
2��0.3mol������������0.1mol����n��C����n��H����n��O��=2��6��1��
�ʴ�Ϊ��n��C����n��H����n��O��=2��6��1��
��2����n��C����n��H����n��O��=2��6��1�������л��������ʵ��ʽΪC
2H
6O���ʴ�Ϊ��C
2H
6O��
��3��A�Ŀ��ܽṹΪ��CH
3CH
2OH��CH
3-O-CH
3���ʴ�Ϊ��CH
3CH
2OH��CH
3-O-CH
3��
��4�����л���A��������3����ԭ�ӣ�����A�Ľṹ��ʽΪ��CH
3CH
2OH���ʴ�Ϊ��CH
3CH
2OH��
��5�����ݴ��ܱ������������Һ��������ȩ������ʽ�ֱ�Ϊ��2CH
3CH
2OH+O
2 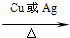
2CH
3CHO+2H
2O��
CH
3CHO+2Ag ��NH
3��
2OH

2Ag��+3NH
3��+H
2O+CH
3COONH
4���ʴ�Ϊ��2CH
3CH
2OH+O
2 
2CH
3CHO+2H
2O��
CH
3CHO+2Ag ��NH
3��
2OH

2Ag��+3NH
3��+H
2O+CH
3COONH
4��
��6����ϩ�ܷ����Ӿ۷�Ӧ���ɾ���ϩ��n CH
2=CH
2 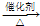

���ʴ�Ϊ��n CH
2=CH
2 

��
��7����ϩ�����Ȼ��ⷢ���Ӿ۷�Ӧ���������飺CH
2=CH
2+HCl

CH
3CH
2Cl���ʴ�Ϊ��CH
2=CH
2+HCl

CH
3CH
2Cl��
��8�������ܺ��Ҵ�����������Ӧ��CH
3COOH+CH
3CH
2OH

CH
3COOC
2H
5+H
2O���ʴ�Ϊ��CH
3COOH+CH
3CH
2OH

CH
3COOC
2H
5+H
2O��
������������Ҫ���������ʵ���ɡ��ṹ�����ʣ��ѶȲ����ݿα�֪ʶ������ɣ�

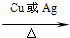 2CH3CHO+2H2O��
2CH3CHO+2H2O�� 2Ag��+3NH3��+H2O+CH3COONH4���ʴ�Ϊ��2CH3CH2OH+O2
2Ag��+3NH3��+H2O+CH3COONH4���ʴ�Ϊ��2CH3CH2OH+O2  2CH3CHO+2H2O��
2CH3CHO+2H2O�� 2Ag��+3NH3��+H2O+CH3COONH4��
2Ag��+3NH3��+H2O+CH3COONH4��
 ���ʴ�Ϊ��n CH2=CH2
���ʴ�Ϊ��n CH2=CH2 
 ��
�� CH3CH2Cl���ʴ�Ϊ��CH2=CH2+HCl
CH3CH2Cl���ʴ�Ϊ��CH2=CH2+HCl CH3CH2Cl��
CH3CH2Cl�� CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+CH3CH2OH  CH3COOC2H5+H2O��
CH3COOC2H5+H2O��


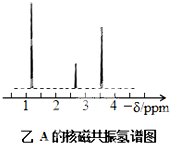 Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮
Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮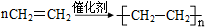
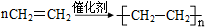
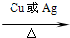 2CH3CHO+2H2O
2CH3CHO+2H2O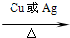 2CH3CHO+2H2O
2CH3CHO+2H2O
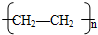 ��
��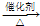
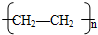 ��
��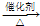 CH3CH2Cl��
CH3CH2Cl��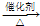 CH3CH2Cl��
CH3CH2Cl�� CH3COOC2H5+H2O��
CH3COOC2H5+H2O�� CH3COOC2H5+H2O��
CH3COOC2H5+H2O��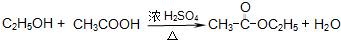
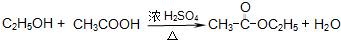
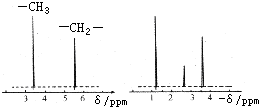 Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮
Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮