
����Ŀ����X��Y��Z��W���ֶ���������Ԫ�أ�ԭ��������������Ԫ��������ԭ�ӣ���������ṹ���±���ʾ��
Ԫ�ر�� | Ԫ��������ԭ�ӣ���������ṹ |
X | ԭ�Ӻ���û������ |
Y | �����µ���Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷ� |
Z | ���ڲ��������������2�� |
W | ������Ԫ����ԭ�Ӱ뾶��С |
��ش�
��1��д��Ԫ��Z�����ڱ��е�λ��______������Ԫ��W��ԭ�ӽṹʾ��ͼ______��
��2��Y��W��ȣ���̬�⻯���ȶ��Խ�������________���ѧʽ����ͬ������Ԫ������������Ӧˮ�������Ը�ǿ����__________��
��3��X��Y��Z����Ԫ�ؿ����γɻ�����ZYX2�����뻯����XW������Ӧ�����������Σ�д���÷�Ӧ�Ļ�ѧ����ʽ________________��
���𰸡���3���ڣ���A�� 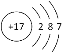 NH3 HClO4 NaNH2+2HCl = NH4Cl+NaCl
NH3 HClO4 NaNH2+2HCl = NH4Cl+NaCl
��������
X��Y��Z��W���ֶ���������Ԫ�أ�ԭ��������������Xԭ�Ӻ���û�����ӣ���XΪ��Ԫ�أ�������Y����Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷԣ���YΪ��Ԫ�أ�ZԪ��ԭ�����ڲ��������������2����Zԭ������������Ϊ1������IA�壬ԭ���������ڵ��ģ���ZΪNa��W�DZ�����Ԫ����ԭ�Ӱ뾶��С��ԭ����������Na����WΪClԪ�����ݴ˽��
(1)ZΪNaԪ�أ������ڱ���λ�ڵ������ڵ�IA�壬 WΪClԪ�أ�ԭ�ӽṹʾ��ͼΪ��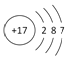 ���ʴ�Ϊ���������ڵ�IA�壻
���ʴ�Ϊ���������ڵ�IA�壻 ��
��
(2)��Ԫ�صķǽ����Աȵ�ǿ����̬�⻯���ȶ��Խ�������NH3��HClO4����ǿ���������ᣬ���Ա������ǿ���ʴ�Ϊ��NH3��HClO4��
(3)������NaNH2���뻯����HCl������Ӧ�����������Σ�Ӧ�����Ȼ������Ȼ�泥���Ӧ�Ļ�ѧ����ʽ��NaNH2+2HCl=NH4Cl+NaCl���ʴ�Ϊ��NaNH2+2HCl=NH4Cl+NaCl��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
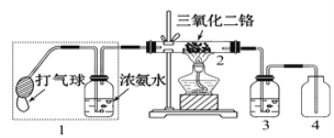
����Ŀ��ij����������Ҫ��Cr2O3��Al2O3��Fe2O3�����ʣ������������ʪ���������ã���������ͼ��ʾ��

��֪�������²������������Ksp���±�
Al(OH)3 | Fe(OH)3 | Cr(OH)3 | Mn(OH)2 | |
Ksp | 3��10-34 | 4��10-38 | 6��10-31 | 4��10-14 |
��1�����ǰ���Ժ����������Ԥ��������ˮʪĥ�ɽ��壬ʪĥ��������_____________��
��2������ҺI��KMnO4����Cr3+�����ӷ���ʽ��________________________________;��Cr3+������Ŀ����____________________________________________________��
��3�������£���pH=10ʱ�� ![]() =_____������ҺpH���ܴ���10��������_________��
=_____������ҺpH���ܴ���10��������_________��
��4��NaHSO3�ڷ�Ӧ�е�������___________��������n(NaHSO3):n[CrOH(H2O)5SO4]֮����_______________��
��5��NaHSO3�����ڿ������ױ��ʣ�д������NaHSO3�Ƿ���ʵķ���______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪʵ������ȡ����ˮ��װ��ʾ��ͼ������ͼʾ�ش��������⡣
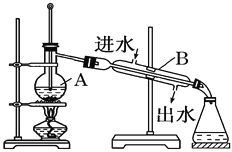
(1)ָ��ͼ���������ԵĴ���
��____________________________����____________________________��
(2)A������������__________��B������������__________��
(3)ʵ��ʱA�г�������������ˮ�⣬�����������__________���������Ƿ�ֹ����ʱҺ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������һ�ֳ����Ļ��ʣ�ij������ʯ�ࡢNH3��H2O��CO2�Ʊ�����淋Ĺ����������£�

����˵������ȷ����
A. ����1Ϊ���ˣ�����2Ϊ����
B. ��Һ�е���Ҫ�����ӿ�ͨ����������Һ�м�NaOH��Һ�����ȣ�����ʪ��ĺ�ɫʯ����ֽ������
C. ���ҷ�Ӧ�����ӷ���ʽΪCaSO4+2NH3+CO2+H2O==CaCO3��+2NH4++ SO42-
D. ���������в�����CO2��ѭ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӧ2H2O2(l)![]() 2H2O(l)+O2(g)�����仯��ͼ��ʾ������˵����ȷ����
2H2O(l)+O2(g)�����仯��ͼ��ʾ������˵����ȷ����

A. ���ڷֽⷴӦ�����Ը÷�Ӧ����
B. ;��b�ı��˷�Ӧ����ЧӦ
C. 1 molH2O2(l)����������1 molH2O(l)������
D. ;��a�ų���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ԫ���ڵ����Ϻ����ḻ�����а����ڹ�ũҵ����������������Ҫ�����á�
��1�������е���_________���������ţ���ʽ���ڡ��̵��ǵ�ѭ������Ҫ���ڣ���ҵ�̵���NH3��Ӧ�¶�ѡ��500�����ҵ�ԭ����________________________________��
��2��ij��ȤС������ͼװ��̽�����Ĵ����������Ȳ�����2һ��ʱ���ѹ1�д��������������۲쵽2�����ʳʺ���״̬��ֹͣ���Ⱥ����ܱ��ֺ��ȣ��÷�Ӧ��_________��Ӧ(��������������������)��
��3����ͼ����ȡ��3����4�н��۲쵽�������̣��ð��̵Ļ�ѧʽ����Ϊ______________��
��4��ʵ���һ�����Ũ��ˮ���������ƹ����ϣ��Ƶð���������ƽ���ƶ���������ص����۽��÷������Ʊ�������ԭ��_____________________________________________��
��5������ͼװ�ý��а���������ʵ�飨����1������2ʵ��ǰ�رգ���G��H��������������������ȴ�����1��һ��ʱ��ر�����1���ٴ�����2��Hƿ��������������________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ������������⣺
�١�  �˼���ʽ����ʾ�л���Ľṹ��ʽ�ǣ�________________��
�˼���ʽ����ʾ�л���Ľṹ��ʽ�ǣ�________________��
�ڡ� ���л����ϵͳ����Ϊ��________________��
���л����ϵͳ����Ϊ��________________��
�ۡ� ![]() ���л�����ϵͳ�������������ǣ�________________��
���л�����ϵͳ�������������ǣ�________________��
�ܡ�д���ṹ��ʽ�� 2,2-����-3-�һ�����________________��
�ݡ�д���ṹ��ʽ�� 2������2����ϩ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�Һ�°������й����Ƽ�գ��ٽ��������Ƽ���ķ�չ����ͼ�Ǵ���յļ���
��1��д��CO2�ĵ���ʽ____________________��
��2�������ӷ���ʽ��ʾ�������HCO3-������___________________��
��3����ҵ����ʱ�Ȱ�����ͨCO2��˳���ܵߵ���ԭ����_______________��
��4����ҺA������Ҫ������������_________��
��5��ijС���������ʵ�������ҺA�е���Ҫ���ʡ���Һ©��������һ��ʱ����Թ����а�ɫ�������ɣ��û�ѧԭ�����Ͱ�ɫ���������ԭ��___________________��

��6��ij������Ʒ�����ղ���ֶ�������NaHCO3��ȡ����Ϊm1�Ĵ�����Ʒ����ּ��Ⱥ�����Ϊm2�������Ʒ��̼�����Ƶ���������Ϊ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪: �����£�(1) Ka1(H2CO3)=4.3��10-7, Ka2(H2CO3)=5.6��10-11��(2)H2R�������ε���Һ�У�H2R��HR-��R2-�ֱ�����������ռ�����ʵ�����������������ҺpH�仯��ϵ��ͼ��ʾ�����������������
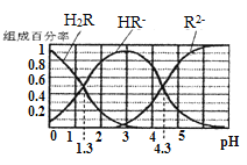
A. ��pH=4.3����Һ�У�3c��R2-��=c��Na+��+c��H+��-c��OH-��
B. �������Ũ�ȵ�NaOH��Һ��H2R��Һ��Ϻ���Һ��ˮ�ĵ���̶ȱȴ�ˮ��
C. ��pH=3����Һ�д���c��R2����c��H2R��/c2��HR��=10-3
D. ��Na2CO3��Һ�м�������H2R��Һ��������Ӧ��2CO32-+H2R=2HCO3-+R2-
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com