
|
��SO2����������Fe2(SO4)3��Һ��ȫ��Ӧ���ټ���K2Cr2O7��Һ����������������ѧ��Ӧ��SO2��2Fe3+��2H2O Cr2O | |
| [����] | |
A�� |
��ԭ��Cr3+��Fe2+��SO2 |
B�� |
������Cr2O |
C�� |
Cr2O |
D�� |
������Ӧ��Fe2(SO4)3������ԭ�� |
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
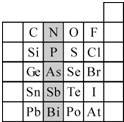 ��1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��
��1������A��B��C��D��E����Ԫ�أ�A��ԭ�Ӻ���û�����ӣ�B��CԪ�ش���ͬһ���ڣ�C��ԭ�Ӱ뾶��С��B��C��������֮��Ϊ27��������֮��Ϊ5��0.96g D�ĵ��ʸ��������ᷴӦ������D3+��1.2L����״����������E��C���γ�E2C�����ӻ������E��C��Ԫ�صļ����Ӿ�����ͬ���Ӳ�ṹ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
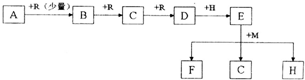
| ||
| ||
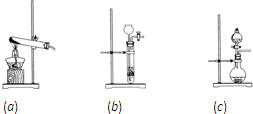
| ||
| ||
| 20V |
| 7 |
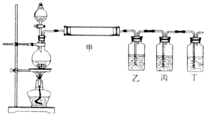
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�������ʵ��Ŀ��������ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���Ĵ�ʡ�����и�����һ���¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ�������ʵ��Ŀ��������ǣ� ��
��� ʵ����� ʵ��Ŀ��
A �ⶨHF��HCl���۵㡢�е� �Ƚ�F��Cl�ķǽ�����ǿ��
B MgSO4��Al2(SO4)3��Һ�зֱ�μ�������ˮ �Ƚ�þ�����Ľ�����ǿ��
C ��SO2����ͨ��̼������Һ�� �Ƚ�̼����ķǽ�����ǿ��
D ���Ȼ�李��Ȼ��������Һ�У��μ�����������Һ������ �Ƚ�NaOH��NH3��H2O��Al(OH)3�ļ���ǿ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com